#cell membranes
Text
New publication out!
We were curious what happens with the lipid membrane if proteins are inside. As a model of membrane proteins we use transmembrane peptides with neutral and positive charge. The charges turn out to be quite crucial in the peptides influence on the lipids.
publication link: https://doi.org/10.1016/j.colsurfb.2024.113765…
All transmembrane peptides hinder mobility of lipids around. The positive charges in the peptide make this hindering a long range influence.
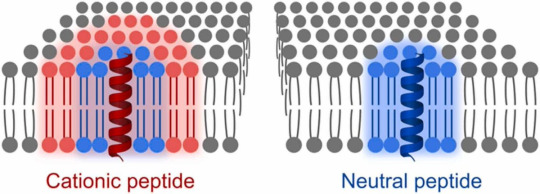
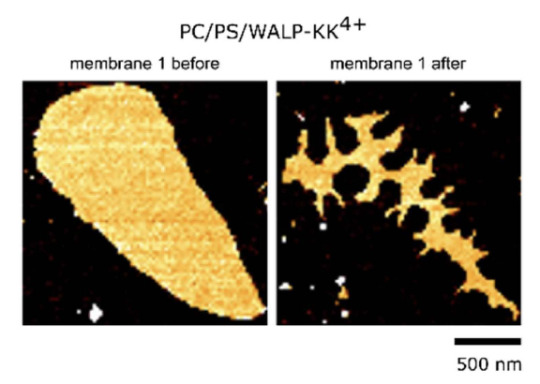
The membrane with the positive peptides then has heterogeneities in the lipid mobility. This hampers free rearrangement of lipids and leads to lower ability to seal ruptures. You can see below how the membrane stays fragmented after indentations with AFM (atomic force microscope), which is basically a very tiny tip that scans the surface of the membrane for images and can also push through it to test mechanical stability.
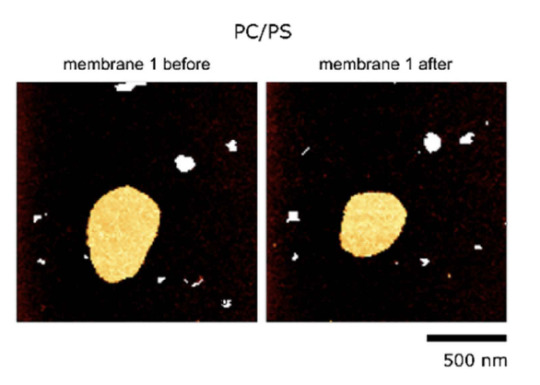
For comparison this is how it looks like with a membrane without any peptides. The mebrane seals the ruptures induced by the AFM tip and recovers its round shape. The same happens if we have a neutral, non-charged, peptide.
With this work we highlight the importance of including charges of proteins and peptides in membrane model studies.
And last but not least...
This is the very first time I am the last corresponding author of a publication. I am immensely proud on myself!
#science#women in science#research#biophysics#afm#fluorescence#simulations#peptides#proteins#cell membranes#publication#collaboration#corresponding author#proud#original content
15 notes
·
View notes
Text
Cross-cell Traffic
Visualising and analysing in live cells how one of the least-well understood members of the family of lipid molecules called PIPs regulates trafficking across cell membranes
Read the published research paper here
Edvard Munch, painter of the acclaimed image The Scream was born on this day (12th December) in 1863
Adapted video from work by James H. Vines and colleagues
School of Biosciences, University of Sheffield, Firth Court Western Bank, Sheffield, UK
Video originally published with a Creative Commons Attribution 4.0 International (CC BY 4.0)
Published in Journal of Cell Biology, June 2023
You can also follow BPoD on Instagram, Twitter and Facebook
#science#biomedicine#biology#dictyostelium#amoeba#lipids#cell signaling#edvard munch#the scream#cell membranes
14 notes
·
View notes
Text
Trapping Sulfate to Benefit Health, Industry and Waterways - Technology Org
New Post has been published on https://thedigitalinsider.com/trapping-sulfate-to-benefit-health-industry-and-waterways-technology-org/
Trapping Sulfate to Benefit Health, Industry and Waterways - Technology Org
Scientists have developed a new method to measure and remove sulfate from water, potentially leading to cleaner waterways and more effective nuclear waste treatments.
Water – illustrative photo. Image credit: Pixabay (Free Pixabay license)
A collaborative team from The University of Queensland and Xiamen University in China has designed a cage-like molecule to trap sulfate, a naturally occurring ion, in water.
Professor Jack Clegg from UQ’s School of Chemistry and Molecular Biosciences said controlling the sulfate concentration in water is a significant challenge in health, industry and environmental management.
“Sulfate is a very common and important ion,” Professor Clegg said.
“In low quantities in the human body, sulfate has diverse metabolic roles such as eliminating toxins and helping drugs work effectively.
“But in the environment, too much sulfate can pollute drinking water and accelerate the corrosion of pipes.
“The presence of sulfate also causes problems when immobilising radioactive wastes.
“Being able to monitor and completely remove sulfate in water has great potential across many areas.”
The researchers developed a molecule that measures and traps sulfate in water with a high degree of selectivity.
This ‘molecular trap’ can be prepared inexpensively from off-the-shelf chemicals.
Dr Xin Wu, a former DECRA fellow at UQ now based at Xiamen University, said while there are enormous benefits from cheaply and easily measuring sulfate levels, the molecular trap’s ability to capture negatively charged chemicals from water is also valuable.
“Being able to stabilise a highly negatively charged chemical such as sulfate inside a charge-neutral cavity is a remarkable feature of our molecule,” Dr Wu said.
“This mimics the function of naturally occurring sulfate-binding proteins.
“The technology could also have applications in medicine, such as helping to funnel chloride and bicarbonate ions through cell membranes to treat diseases that involve defective ion transport such as cystic fibrosis.
“This is just the beginning – we’re excited to see how this fundamental science can be applied in all sorts of fields.”
The research paper is published in Nature Chemistry.
Source: University of Queensland
You can offer your link to a page which is relevant to the topic of this post.
#applications#Capture#cell#Cell membranes#challenge#chemical#chemicals#chemistry#Chemistry & materials science news#China#collaborative#corrosion#cystic fibrosis#Diseases#drinking#drinking water#drugs#Environment#Environmental#Featured technology news#fibrosis#Fundamental#Health#how#human#Industry#Link#management#measure#Medicine
1 note
·
View note
Photo

(via Saturated Fats: The Good, the Bad, and the Ugly)
Saturated fats have gotten bad press over the past few decades, but they’re not all created equal. Some saturated fats are actually good for you, while others may increase your risk of heart disease and other health problems.
#cell membranes#cholesterol#fats#health problems#heart disease#hormone imbalance#metabolism#olive oil#protein synthesis#saturated fats
0 notes
Text
Absorption of light allows 11-cis-retinal to relax from a bent shape (arising from the cis double bond) to a lower energy, all-trans arrangement. The straighter all-trans-retinal can no longer fit in the receptor designed for 11-cis-retinal and is ejected. This change leads to differences in cell membrane potential and, as ions are pumped into the cell, this response turns into a nerve impulse that travels to the brain. That is not the end of the matter; the all-trans-retinal generated by this process is reconverted to 11-cis-retinal by specific enzymes, which can re-bind into the receptor, ready to interact with another photon of light.
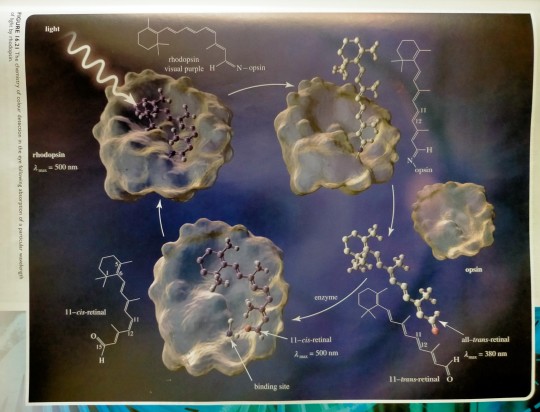
FIGURE 16.21 The chemistry of colour detection in the eye following absorption of a particular wavelength of light by rhodopsin.
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#light absorption#light#wavelength#rhodopsin#cis#trans#cell membrane#retinal#receptor#enzyme#opsin#photon#straight#oops all trans
38 notes
·
View notes
Text

I seriously haven’t been normal ever since I found out about the Zimvoid arc
#iz zib#iz meme#zimvoid#iz comics#zib membrane#invader zib#?#iz comic spoilers#my memes#look at this greasy little roach boy#oh my god#this nasty little twerp#this feral nerd#this aberrant science goblin#living proof that zim and dib have less than one brain cell between them#scrungly blorbo
26 notes
·
View notes
Text
Cell membrane has a BI-layer of lipids?
YOU KNOW WHAT. THIS LITERALLY EXPLAINS MY VERY BEING.
#biology#cbse#ncert#desi#studyblr#school#students#desiblr#desi academia#chaotic academia#academia#dark academia#cell membrane#bisexual#biromantic#lgbtq
73 notes
·
View notes
Text
How to learn biology diagrams
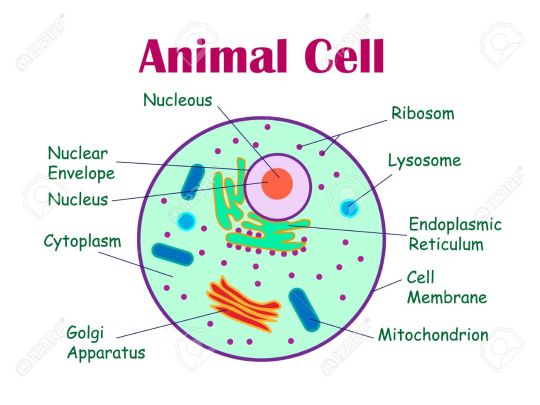
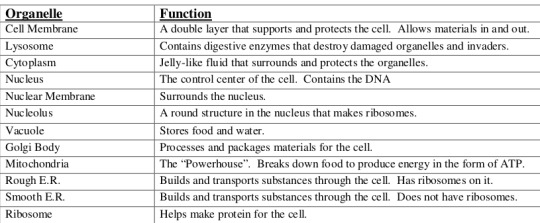
For example this is the diagram you have to learn so you can learn it like-
Each cell has a protective outer layer (plasma membrane) , so the cell only lets some certain things or nutrients into the system that it needs but keeps other things out (semipermeable).
Inside the animal cell there is the digestive system (cytoplasm), in the digestive system there is the working organs (organelles).
Tiny grains floating inside the system are called (ribosome) where protein is made.
The cell also has a DNA (nucleus) that contains all are genetic information the DNA is found on structures of the cell (nucleus) called chromosomes .
The nucleus is surrounded by a layer or cover (nuclear membrane) which controls what goes in and out.
Rough ER is a series of folded protective outer layer (membrane) rough ER is always spotted with the tiny grains floating around the system (ribosomes). Together the ribosomes and rough ER make new proteins and protective layer (membranes) that the system or cell needs.
While smooth ER is the opposite, it has vehicles which are used to move things around the digestive system (transport vesicles).
Now there is another working organ which helps in packaging up things, to be transported around cells or need to leave the cell like hormones (Golgi Apparatus).
Now comes lysosomes, lysosomes are both the vitamin c and the decomposer of the cell. It kills the bacteria that invades the body and it also break downs the THINGS that cell doesn 't need.
Vacuoles are large membranous sacs used to store things. While vesicles are smaller sacs.
Mitochondria is the powerhouse that breaks down sugar (ATP) which helps the cell get energy .
Made by Advika
#biology diagrams#diagrams#biology#memory#How to learn biology diagrams#rough er#smooth er#golgi apparatus#vacuoles#vesicles#mitochondria#lysosomes#cytoplasm#ribosomes#membrane#cell#animal#animal cell#system
4 notes
·
View notes
Text
Obi-Wan Gets Ready to Rejoin the Duel

STAR WARS EPISODE I: The Phantom Menace 01:56:01
#Star Wars#Episode I#The Phantom Menace#Naboo#Theed#Battle of Theed#Battle of Naboo#Plasma Refinery Complex#Duel of the Fates#Obi-Wan Kenobi#Obi-Wan Kenobi's lightsaber#Padawan braid#power cell reserve cap#Jedi tunic#acceleration shaft#thermal carbon membrane
2 notes
·
View notes
Text
The Newcomer
From @ghouljams cod fae!au, Mal gets bugged by someone new.
Mal sat on the floor of their shop, large stone mortar and pestle between their legs as they ground madder roots for dyeing. Their mind was carefully blank, constructing the most neutral emotional state as possible, so as not to impart any one particular intent on the dye goods. In a lot of cases, Mal had to harvest and process things prior to knowing what they would actually be used for. This meant they’d had plenty of practice over the years in imparting as neutral an intent as possible on the goods they kept stocked.
The sound of the madder root slowly grinding into a powder against the aged stone was a familiar one, and Mal could pick out exactly when the powder was good enough by sound alone, going gradually from the popping and crushing of whole roots to the gentle hiss of smooth powder between ancient stones. But it wasn’t there yet.
Mal felt a presence brush against the open curtains outside their shop, before there was a gentle knock at the door. They were in the zone though, mind blissfully blank, and felt no urgency to get the door. Afterall, the madder wasn’t finished yet. Large chunks still remained interspersed amongst the finer powder, which just wouldn’t do.
By the time they were done, enough time had passed to cause the shadows to noticeably shift in the shop. They only felt a little bad at the prospect of having lost a customer, afterall their commission log stayed quite full these days. Cleaning up, Mal poured the fresh madder into its glass jar and found a spot for it on the large, over cluttered shelves along the wall of the shop. Preserved and processed dye plants from all over the world found their cozy home among these shelves.
They felt the gentle brush of a presence against the wards of their shop once again, making them jump slightly. It felt familiar, like the one from earlier in the day, but that seemed unreasonable. Who would have waited this long? Witch could let herself in, and this felt different from that codependent pair, Love and Ghost.
When Mal opened the door, they saw a handsome fae idly playing with the fabric of the exterior shop curtains. At the sound of the door he whipped around, as if caught with his hand in the cookie jar, and smiled sheepishly.
“Sorry about that, the craftsmanship is just amazing I couldn’t help myself. Did you make these?”
“I did,” Mal said, “are you the one from earlier?”
“Oh yeah, I didn’t mind waiting though, you seemed busy.”
Huh. “Are you looking to come in?”
His smile brightened, “If you don’t mind. I’ve heard such good things about your work.”
“You’re welcome in, for this transaction,” Mal said, opening the door wider and feeling the ward surround him like a bubble as he slipped inside. He looked around the shop with wonder, full to the brim with textiles, fiber, dyestuffs, and more. Seemingly forgetting they were there, he strolled around the shop gently touching and admiring everything on display.
Eventually Mal’s patience wore out. They cleared their throat, losing their train of thought for a second when he swiftly turned his head, giving them his full attention. His eyes were a warm brown, almost yellowish in the afternoon light, and his gaze felt heavy with. . .something.
Quickly recovering, they said, “So, did you have something you were looking for?” Mal really wanted to say ‘What do you want’, but decades spent getting coached by friends on ‘social niceties’ taught them that that would seem ‘rude’. He joined Mal at the high counter top that doubled as a crafting and consultation station, resting his elbows against it and settling in. His eyes were even more brilliant up close.
He sighed through his nose, pursing his lips in thought before saying, “No, not really.”
Mal’s eyebrows furrowed, “What?”
He shrugged, “There’s not really anything I’m looking for right now.”
“Then why did you come in? Don’t you have something you want? A new obscura, protective wear?”
“Nope,” he said, popping the ‘p’. A small smile bloomed on his face, seemingly amused at their confusion.
“Then why are you here?”
He swung his arms open wide, once again taking in the splendor of the shop, “Like I said, I’ve heard such good things about you and your work, and I've peered into your shop once or twice while I've been out and about. I just had to come in and. . .”
Once again he dropped down onto his elbows, leaning over the counter slightly. His eyes quickly flickered up and down, before staring straight into their eyes, “. . .see what all the fuss is about.”
Mal hummed, oblivious to the once over and took a look at the clock, “Well, I hope your perusal was satisfactory, the shop is closing soon. For future reference it would be helpful to have some kind of idea when you come in.”
As the clock struck the hour, the ward that had bubbled around the newcomer constricted slightly and his eyes briefly flashed with shock and alarm. It wasn’t a dangerous pressure (yet), but it was uncomfortable and seemed to be pressing most towards the direction of the door.
He flashed Mal a dashing smile as he walked backwards towards the door, aided by the ward to keep him from knocking anything over, “I’ll be seeing you around then.”
He winked, and the door shut behind him with a satisfying click.
#1fae1#oc: mal#cod au#maelstrom007#maelstrom fic#maelstrom writes#Mal gets bugged#Cause I say so#i'm not really sure who this fae is yet#he needs a name#he just sprung out of my head as i was writing#i like him though#i think the wards on the shop behave like how things move in and out of cells#yknow how they go up to the membrane#and then the membrane forms a bubble around them and lets them in#i like it cause i think itd be a very effective way to kick someone out like how Mal did#and maybe squish them into a pulp#as a treat#Mal is so oblivious#next time Mal talks with Witch shes gonna be like#Mal they were flirting with you#Mal: Pikachu face
6 notes
·
View notes
Link
Proton-exchange membrane fuel cells (PEMFC), which are being developed for use in electric vehicles, rely on nanoparticles called catalysts to trigger electricity-producing reactions between hydrogen and oxygen. Most PEMFC catalysts contain platinum -- a scarce and precious metal. There is therefore a pressing global need to develop catalysts that can generate the most power while minimizing platinum content.
Manufacturers integrate these catalysts in complex assemblies called catalyst layers. Until now, they had to do so without a detailed picture of the resulting structure, as traditional imaging processes almost always cause some degree of damage. Vasiliki Tileli, head of the Laboratory for in-situ nanomaterials characterization with electrons in the School of Engineering, has found a way around this challenge. By imaging catalysts and their environment at below-freezing temperatures using cryogenic transmission electron tomography and processing the images with deep learning, she and her colleagues have succeeded in revealing, for the first time, the nanoscale structure of catalyst layers.
"We're still far away from PEMFCs without platinum, which is very expensive, so in the short term, we need to reduce platinum loading to make this technology viable for mass production. It's therefore imperative to understand how platinum sits in relation to other materials within the catalyst layer, to increase the surface area contact required for chemical reactions to take place," Tileli explains.
Read more.
#Materials Science#Science#Fuel cells#Membranes#Nanoparticles#Catalysts#Platinum#Nanotechnology#Surfaces#Reactions#Materials Characterization#Cryogenics
9 notes
·
View notes
Photo

Partial Breakdown
Like too much of a good thing, high levels of cholesterol, an essential component of cell membranes, are linked to multiple health problems; pictured are cholesterol crystals in joint or synovial fluid, a symptom associated with diseases like gout. To maintain a healthier balance, cells slow down cholesterol production when it becomes too abundant, by breaking down a key enzyme involved in making cholesterol, squalene monooxygenase (SM). Yet when cells experience low oxygen levels, or hypoxia, this process fails: SM is only partially broken down, leaving a shortened version that remains active, and so keeping cholesterol levels high. Making cholesterol is an oxygen-hungry process, requiring eleven molecules of oxygen for each cholesterol produced, so preserving SM activity when oxygen is low might help cells cope with fluctuations in oxygen. Conversely, interfering with this partial breakdown of SM could potentially be a winning strategy for treatments aiming to bring cholesterol down.
Written by Emmanuelle Briolat
Image by Ed Uthman
Research by Hudson W Coates et al, School of Biotechnology and Biomolecular Sciences, UNSW Sydney, Sydney, Australia
Image originally published with a Creative Commons Attribution 2.0 Generic (CC BY 2.0)
Published in eLife, January 2023
You can also follow BPoD on Instagram, Twitter and Facebook
2 notes
·
View notes
Text
Cells’ Electric Fields Keep Nanoparticles at Bay, Scientists Confirm - Technology Org
New Post has been published on https://thedigitalinsider.com/cells-electric-fields-keep-nanoparticles-at-bay-scientists-confirm-technology-org/
Cells’ Electric Fields Keep Nanoparticles at Bay, Scientists Confirm - Technology Org
The surprisingly strong effect could have implications for drug design and delivery.
The humble membranes that enclose our cells have a surprising superpower: They can push away nano-sized molecules that happen to approach them. A team including scientists at the National Institute of Standards and Technology (NIST) has figured out why, by using artificial membranes that mimic the behavior of natural ones. Their discovery could make a difference in how we design the many drug treatments that target our cells.
Cell membranes generate powerful electric field gradients that are largely responsible for repelling nano-sized particles like proteins from the surface of the cell — a repulsion that notably affects uncharged nanoparticles. In this schematic drawing, a negatively charged membrane (at top, in red) attracts small, positively charged molecules (purple circles), which crowd the membrane and push away a far larger, neutral nanoparticle (pink). Credit: N. Hanacek/NIST
The team’s findings, which appear in the Journal of the American Chemical Society, confirm that the powerful electrical fields that cell membranes generate are largely responsible for repelling nanoscale particles from the surface of the cell. This repulsion notably affects neutral, uncharged nanoparticles, in part because the smaller, charged molecules the electric field attracts crowd the membrane and push away the larger particles. Since many drug treatments are built around proteins and other nanoscale particles that target the membrane, the repulsion could play a role in the treatments’ effectiveness.
The findings provide the first direct evidence that the electric fields are responsible for the repulsion. According to NIST’s David Hoogerheide, the effect deserves greater attention from the scientific community.
“This repulsion, along with the related crowding that the smaller molecules exert, is likely to play a significant role in how molecules with a weak charge interact with biological membranes and other charged surfaces,” said Hoogerheide, a physicist at the NIST Center for Neutron Research (NCNR) and one of the paper’s authors. “This has implications for drug design and delivery, and for the behavior of particles in crowded environments at the nanometer scale.”
Membranes form boundaries in nearly all kinds of cells. Not only does a cell have an outer membrane that contains and protects the interior, but often there are other membranes inside, forming parts of organelles such as mitochondria and the Golgi apparatus. Understanding membranes is important to medical science, not least because proteins lodged in the cell membrane are frequent drug targets. Some membrane proteins are like gates that regulate what gets into and out of the cell.
The region near these membranes can be a busy place. Thousands of types of different molecules crowd each other and the cell membrane — and as anyone who has tried to push through a crowd knows, it can be tough going. Smaller molecules such as salts move with relative ease because they can fit into tighter spots, but larger molecules, such as proteins, are limited in their movements.
This sort of molecular crowding has become a very active scientific research topic, Hoogerheide said, because it plays a real-world role in how the cell functions. How a cell behaves depends on the delicate interplay of the ingredients in this cellular “soup.” Now, it appears that the cell membrane might have an effect too, sorting molecules near itself by size and charge.
“How does crowding affect the cell and its behavior?” he said. “How, for example, do molecules in this soup get sorted inside the cell, making some of them available for biological functions, but not others? The effect of the membrane could make a difference.”
While researchers commonly use electric fields to move and separate molecules — a technique called dielectrophoresis — scientists have paid scant attention to this effect at the nanoscale because it takes extremely powerful fields to move nanoparticles. But powerful fields are just what an electrically charged membrane generates.
“The electric field right near a membrane in a salty solution like our bodies produce can be astoundingly strong,” Hoogerheide said. “Its strength falls off rapidly with distance, creating large field gradients that we figured might repel nearby particles. So we used neutron beams to look into it.”
Neutrons can distinguish between different isotopes of hydrogen, and the team designed experiments that explored a membrane’s effect on nearby molecules of PEG, a polymer that forms chargeless nano-sized particles. Hydrogen is a major constituent of PEG, and by immersing the membrane and PEG into a solution of heavy water — which is made with deuterium in place of ordinary water’s hydrogen atoms — the team could measure how closely the PEG particles approached the membrane. They used a technique known as neutron reflectometry at the NCNR as well as instruments at Oak Ridge National Laboratory.
Together with molecular dynamics simulations, the experiments revealed the first-ever evidence that the membranes’ powerful field gradients were the culprit behind the repulsion: The PEG molecules were more strongly repelled from charged surfaces than from neutral surfaces.
While the findings do not reveal any fundamentally new physics, Hoogerheide said, they do show well-known physics in an unexpected place, and that should encourage scientists to take notice — and explore it further.
“We need to add this to our understanding of how things interact at the nanoscale,” he said. “We’ve demonstrated the strength and significance of this interaction. Now we need to investigate how it affects these crowded environments where so much biology happens.”
Paper: M. Aguilella-Arzo, D.P. Hoogerheide, M. Doucet, H. Wang and V.M. Aguilella. Charged biological membranes repel large neutral molecules by surface dielectrophoresis and counterion pressure. Journal of the American Chemical Society. Published online Jan. 16, 2024. DOI: 10.1021/jacs.3c12348
Source: NIST
You can offer your link to a page which is relevant to the topic of this post.
#2024#approach#artificial#atoms#attention#Behavior#Biology#cell#Cell membranes#Cells#chemical#Chemistry & materials science news#circles#Community#Design#drug#dynamics#electric field#Energy & fuel news#Featured physics news#form#Forms#gradients#how#hydrogen#interaction#isotopes#it#Link#measure
0 notes
Photo
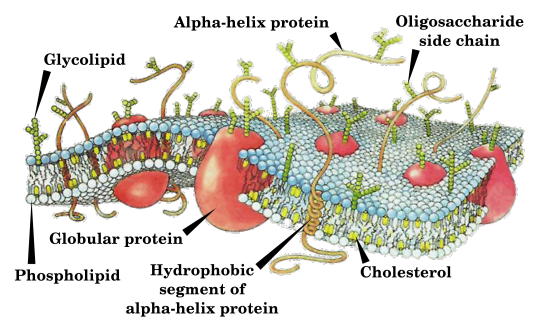
27 notes
·
View notes
Text
Drawing together our information for the cereal aleurone system (Figure 18.11), we can hypothesize that binding of bioactive gibberellin to GID1 leads to degradation of the DELLA protein.
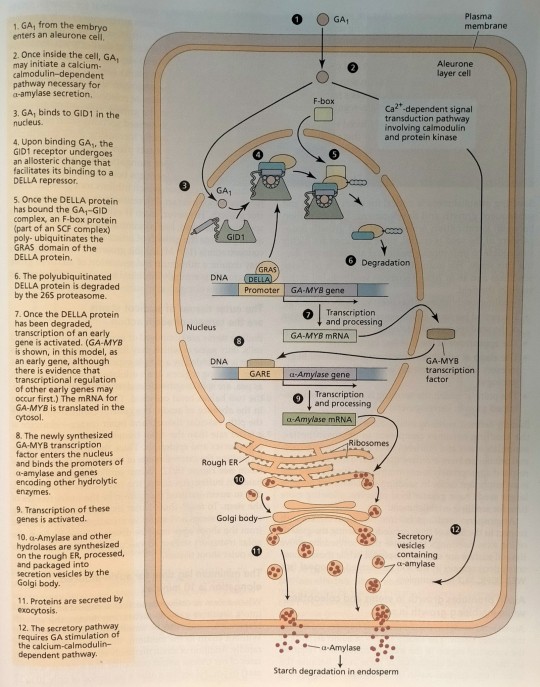

"Plant Physiology and Development" int'l 6e - Taiz, L., Zeiger, E., Møller, I.M., Murphy, A.
#book quotes#plant physiology and development#nonfiction#textbook#gid1#gibberellin insensitive dwarf#della#aleurone#cereals#transcription#plant cells#plasma membrane#alpha amylase#endoplasmic reticulum#ribosomes#golgi body#vesicles#nucleus
2 notes
·
View notes











