#Teriflunomide
Link
Aubagio: A Breakthrough in Multiple Sclerosis Treatment Multiple sclerosis (MS) is a complex neurological condition that affects millions of people worldwide. For years, finding effective treatments for MS has been a medical challenge. However, recent advancements have led to breakthroughs, and one such breakthrough is Aubagio. In this article, we will explore Aubagio's role in transforming the management of multiple sclerosis, its mechanisms of action, and the benefits it offers to patients. What is Aubagio? Aubagio, also known by its generic name Teriflunomide, is an oral medication approved by the FDA for the treatment of relapsing forms of multiple sclerosis. It falls under the category of disease-modifying therapies (DMTs), specifically designed to slow down the progression of MS and reduce the frequency of relapses. [caption id="attachment_53905" align="aligncenter" width="1400"] aubagio[/caption] How Does Aubagio Work? Understanding how Aubagio works is essential to appreciate its impact on multiple sclerosis. Aubagio's active ingredient, Teriflunomide, targets the immune system, which plays a pivotal role in the development and progression of MS. Teriflunomide works by inhibiting specific immune cells called lymphocytes, particularly T-lymphocytes. These immune cells are known to attack the central nervous system in individuals with MS, leading to inflammation and damage to nerve fibers. Benefits of Aubagio Aubagio has garnered attention within the medical community and among MS patients for several compelling reasons: Efficacy in Reducing Relapses One of the primary goals of MS treatment is to reduce the frequency and severity of relapses. Aubagio has demonstrated its effectiveness in achieving this goal. Clinical trials have shown that patients taking Aubagio experienced a significant decrease in relapse rates compared to those on a placebo. Slowing Disease Progression Slowing down the progression of MS is crucial to preserving a patient's quality of life. Aubagio has shown promise in this regard as well. By modulating the immune response and reducing inflammation, it helps protect nerve fibers from further damage. Oral Administration Aubagio stands out as an oral medication in the realm of MS treatments. Unlike injectable therapies, which some patients may find challenging, Aubagio is taken orally in tablet form. This convenience can lead to better adherence to the prescribed treatment plan. Favorable Side Effect Profile While all medications carry potential side effects, Aubagio is known for its relatively mild side effect profile. Common side effects may include mild hair thinning, diarrhea, or elevated liver enzymes. These effects are usually manageable and tend to subside with continued use. Long-Term Safety Aubagio's safety and tolerability have been studied extensively over the years. Long-term studies have shown that it maintains its efficacy and safety even with prolonged use, providing patients with a reliable treatment option. Side Effects and Precautions While Aubagio offers significant benefits in managing multiple sclerosis, it's essential to be aware of potential side effects and take necessary precautions when using this medication. Potential Side Effects Mild Hair Thinning: Some individuals may experience mild hair thinning while taking Aubagio. This side effect is usually temporary and reversible upon discontinuation of the medication. Diarrhea: Diarrhea is a common side effect of Aubagio. It is advisable to stay hydrated and consult your healthcare provider if diarrhea becomes severe or persistent. Elevated Liver Enzymes: Periodic monitoring of liver function is recommended while on Aubagio. Elevated liver enzymes may occur in some cases, but they are typically reversible upon dose adjustment or discontinuation. Infections: Aubagio may increase the risk of certain infections. It's crucial to promptly report any signs of infection, such as fever or persistent sore throat, to your healthcare provider. Precautions To ensure safe and effective use of Aubagio, consider the following precautions: Regular Monitoring: Follow your healthcare provider's recommendations for routine monitoring of your overall health, including liver function tests and blood cell counts. Pregnancy and Contraception: Aubagio may cause birth defects if used during pregnancy. If you are of childbearing age, discuss effective contraception methods with your healthcare provider. Vaccinations: Keep up-to-date with recommended vaccinations before starting Aubagio, as some vaccines may need to be administered before treatment. Interactions: Inform your healthcare provider about all the medications and supplements you are taking to check for potential interactions with Aubagio. Aubagio vs. Other MS Treatments When it comes to managing multiple sclerosis, patients have several treatment options available. Understanding how Aubagio compares to other treatments can help individuals make informed decisions about their healthcare. Aubagio vs. Injectable Therapies Traditionally, injectable therapies like interferon beta and glatiramer acetate have been common choices for MS treatment. While effective, some patients may find the idea of injections less appealing. Aubagio offers a convenient alternative as an oral medication, potentially improving adherence for those who prefer not to self-administer injections. Aubagio vs. Infusion Therapies Infusion therapies, such as natalizumab and ocrelizumab, involve administering medication intravenously in a clinical setting. While these treatments can be highly effective, they require regular visits to a healthcare facility. Aubagio, on the other hand, allows for self-administration at home, offering greater flexibility for patients. Aubagio vs. Siponimod Siponimod is another oral medication approved for relapsing forms of MS. Both Aubagio and Siponimod work by modulating the immune system, but they have different mechanisms of action. Your healthcare provider can help determine which of these medications may be more suitable based on your specific health needs. Future Developments and Research The field of multiple sclerosis treatment is continually evolving, with ongoing research aimed at enhancing the effectiveness and accessibility of therapies like Aubagio. Here are some key areas of focus for future developments: Novel Therapies Researchers are exploring novel treatment approaches that target different aspects of MS, such as neuroprotection and myelin repair. These therapies aim to complement existing treatments like Aubagio and further improve outcomes for patients. Personalized Medicine Advancements in genetic research are paving the way for personalized medicine in MS treatment. Tailoring treatments to an individual's genetic makeup may lead to more precise and effective therapies. Digital Health Solutions Digital health tools, including mobile apps and wearable devices, are being developed to help patients monitor their MS symptoms and medication adherence more effectively. These technologies aim to empower patients to manage their condition. Expanded Access Efforts are underway to improve access to MS treatments, ensuring that more individuals can benefit from medications like Aubagio. This includes addressing affordability and healthcare disparities. Clinical Trials Participation in clinical trials is essential for evaluating new MS treatments. Patients interested in contributing to research and accessing potentially groundbreaking therapies should consider involvement in clinical trials. Frequently Asked Questions (FAQs) About Buerger's Disease 1. What is Buerger's disease? Buerger's disease, also known as thromboangiitis obliterans, is a rare and serious condition that affects blood vessels in the arms and legs. It causes inflammation, blood clot formation, and blockages in these vessels, leading to reduced blood flow. 2. What are the common symptoms of Buerger's disease? The typical symptoms include pain and numbness in the affected limbs, cold extremities, skin discoloration, and the development of painful sores or ulcers on the fingers and toes. 3. Who is at risk of developing Buerger's disease? Buerger's disease is strongly associated with tobacco use, particularly smoking. Individuals who smoke or use tobacco products are at a significantly higher risk of developing this condition. It is rare in non-smokers. 4. How is Buerger's disease diagnosed? Diagnosis often involves a physical examination, medical history review, and imaging tests like angiography. Doctors may also perform blood tests and vascular studies to assess blood flow. 5. Can Buerger's disease be cured? The most effective treatment for Buerger's disease is complete tobacco cessation. If a patient continues to use tobacco, the disease typically progresses. Smoking cessation programs and support are essential for managing the condition. 6. What treatments are available for Buerger's disease? Aside from quitting smoking, treatments may include medications to alleviate symptoms, wound care for ulcers, and in some cases, surgery to remove damaged blood vessels or improve blood flow. 7. Is Buerger's disease linked to other health conditions? Buerger's disease is primarily associated with smoking. It is not directly linked to other common vascular conditions like atherosclerosis. However, patients with Buerger's disease should be monitored for other cardiovascular risk factors. 8. Can Buerger's disease lead to amputations? In severe cases where blood flow to the limbs is severely compromised, amputation may become necessary. This underscores the importance of early diagnosis and smoking cessation. 9. Is Buerger's disease a common condition? Buerger's disease is relatively rare in comparison to other vascular diseases. Its prevalence is higher in regions where smoking or tobacco use is widespread. 10. Can Buerger's disease recur after quitting smoking? In some cases, Buerger's disease can recur if a patient resumes smoking or tobacco use. Continued abstinence from tobacco is crucial to prevent disease recurrence. Conclusion In the world of multiple sclerosis treatment, Aubagio shines as a beacon of hope for patients seeking effective, convenient, and well-tolerated options. Its ability to reduce relapses, slow disease progression, and offer a preferable oral administration method has made it a compelling choice for many individuals living with MS.
#Aubagio#Aubagio_benefits#Aubagio_side_effects#Aubagio_therapy#Disease_modifying_therapy#MS_drug_Aubagio#MS_treatment#multiple_sclerosis_drug#Oral_medication#Relapsing_remitting_MS#Teriflunomide#Teriflunomide_medication
0 notes
Text
Epstein-Barr Virus Drug Market Size, Share, Trends, Demand, Growth and Competitive Analysis
Epstein-Barr Virus Drug Market research report has been prepared with the systematic gathering and evaluation of market information for industry which is presented in a form that explains various facts and figures to the business. Report saves valuable time as well as adds credibility to the work that is performed to grow business. This quality report has been planned with full commitment and transparency in research and analysis. With the systematic insights of this report, companies can self-assuredly take decisions about the production and marketing strategies. Epstein-Barr Virus Drug Market document provides the same by studying the market and the industry with respect to numerous aspects.
Epstein-Barr Virus Drug Market report gives explanation about the different segments of the market analysis which is demanded by today’s businesses. Key players are taking actions such as developments, product launches, acquisitions, mergers, joint ventures and competitive analysis in the industry. All the market aspects are estimated and analysed by a team of innovative, enthusiastic and motivated researchers and analysts so that nothing lefts uncovered in the report. Global Epstein-Barr Virus Drug Market research report, it becomes easy to figure out brand awareness and insight about the brand and product among potential customers.
Epstein–Barr virus-associated Lymphomas and Others), Treatment Type (Preventive Treatment, Symptomatic Treatment and Others), Drugs (Acyclovir, Leflunomide/ Teriflunomide and Others), Route of Administration (Oral, Parenteral and Others), End Users (Hospitals, Homecare, Specialty Clinics and Others), Country (U.S., Canada, Mexico, Peru, Brazil, Argentina, Rest of South America, Germany, Italy, U.K., France, Spain, Netherlands, Belgium, Switzerland, Turkey, Russia, Hungary, Lithuania, Austria, Ireland, Norway, Poland, Rest of Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Vietnam, Rest of Asia Pacific, South Africa, Saudi Arabia, U.A.E, Kuwait, Israel, Egypt, Rest of Middle East and Africa) Industry Trends and Forecast to 2028
Access Full 350 Pages PDF Report @
The report provides insights on the following pointers:
Market Penetration: Comprehensive information on the product portfolios of the top players in the Epstein-Barr Virus Drug Market.
Product Development/Innovation: Detailed insights on the upcoming technologies, R&D activities, and product launches in the market.
Competitive Assessment: In-depth assessment of the market strategies, geographic and business segments of the leading players in the market.
Market Development: Comprehensive information about emerging markets. This report analyzes the market for various segments across geographies.
Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the Epstein-Barr Virus Drug Market.
Global Epstein-Barr Virus Drug Market survey report analyses the general market conditions such as product price, profit, capacity, production, supply, demand, and market growth rate which supports businesses on deciding upon several strategies. Furthermore, big sample sizes have been utilized for the data collection in this business report which suits the necessities of small, medium as well as large size of businesses. The report explains the moves of top market players and brands that range from developments, products launches, acquisitions, mergers, joint ventures, trending innovation and business policies.
The following are the regions covered in this report.
North America [U.S., Canada, Mexico]
Europe [Germany, UK, France, Italy, Rest of Europe]
Asia-Pacific [China, India, Japan, South Korea, Southeast Asia, Australia, Rest of Asia Pacific]
South America [Brazil, Argentina, Rest of Latin America]
The Middle East & Africa [GCC, North Africa, South Africa, Rest of the Middle East and Africa]
This study answers to the below key questions:
What are the key factors driving the Epstein-Barr Virus Drug Market?
What are the challenges to market growth?
Who are the key players in the Epstein-Barr Virus Drug Market?
What are the market opportunities and threats faced by the key players?
The major players covered in the Epstein-Barr virus drug market report are GlaxoSmithKline plc, Bristol-Myers Squibb Company, Novo Nordisk A/S, B. Braun Melsungen AG, AstraZeneca, Atara Biotherapeutics, Inc., Biotron Limited, Kuur Therapeutics., EUTILEX., Genocea, Lion TCR Pte. Ltd., Omeros Corporation, ViroStatics, Vironika, LLC, AlloVir, Tessa Therapeutics Ltd.., Viracta Therapeutics, Inc., Seagen Inc., Biogen., Amgen Inc., Gilead Sciences, Inc., and Incyte. among other domestic and global players. Market share data is available for Global, North America, Europe, Asia-Pacific (APAC), Middle East and Africa (MEA), and South America separately. DBMR analysts understand competitive strengths and provide competitive analysis for each competitor separately.
Browse Trending Reports:
Medical Clothing Market Size, Share, Trends, Opportunities, Key Drivers and Growth Prospectus https://www.databridgemarketresearch.com/reports/global-medical-clothing-market
Healthcare Facilities Management Market Size, Share, Trends, Industry Growth and Competitive Analysis https://www.databridgemarketresearch.com/reports/global-healthcare-facilities-management-market
Telehealth Market Size, Share, Trends, Growth Opportunities and Competitive Outlook https://www.databridgemarketresearch.com/reports/global-telehealth-market
Digital Health Technologies Market Size, Share, Trends, Demand, Growth, Challenges and Competitive Outlook https://www.databridgemarketresearch.com/reports/global-digital-health-technologies-market
Active Pharmaceutical Ingredient Api Market Size, Share, Key Drivers, Trends, Challenges and Competitive Analysis https://www.databridgemarketresearch.com/reports/global-active-pharmaceutical-ingredient-api-market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 888 387 2818
UK: +44 208 089 1725
Hong Kong: +852 8192 7475
Email: [email protected]
#Epstein-Barr Virus Drug Market Size#Share#Trends#Demand#Growth and Competitive Analysis#market report#market size#market trends#marketresearch#marketreport#market analysis#markettrends#market share#market research
0 notes
Text
PROMISING THERAPIES AND POTENTIAL CURES FOR MULTIPLE SCLEROSIS

Multiple sclerosis (MS) is a chronic autoimmune disease that affects the central nervous system, specifically the brain and spinal cord. It is characterized by the immune system attacking the protective covering of nerve fibers, known as myelin, leading to inflammation, demyelination (loss of myelin), and a range of neurological symptoms. While there is currently no known cure for MS, there are several promising therapies and potential treatments that aim to manage symptoms, slow disease progression, and improve the quality of life for individuals with MS.
Disease-Modifying Therapies (DMTs): These treatments are designed to modify the course of the disease by reducing the frequency and severity of relapses, slowing down disability progression, and delaying the accumulation of new lesions in the brain and spinal cord. There are several classes of DMTs, including interferons, glatiramer acetate, oral immunomodulators, and monoclonal antibodies. Some examples of DMTs include:
Interferons: These are proteins that help regulate the immune system. They can reduce inflammation and the frequency of relapses. Examples include interferon beta-1a and interferon beta-1b.
Glatiramer Acetate: This is a synthetic protein that mimics myelin basic protein, helping to divert the immune system's attack away from the actual myelin.
Oral Immunomodulators: Drugs like fingolimod, siponimod, and teriflunomide modulate immune responses and reduce inflammation. They are taken orally.
Monoclonal Antibodies: Medications like ocrelizumab, alemtuzumab, and natalizumab target specific immune cells or molecules involved in the disease process.
Stem Cell Therapy: Stem cell transplantation involves replacing the immune system with healthy stem cells derived from the patient's own bone marrow or from a donor. This treatment aims to "reset" the immune system and halt the autoimmune attack on myelin. Autologous hematopoietic stem cell transplantation (aHSCT) has shown promising results in clinical trials for aggressive forms of relapsing-remitting MS.
B-cell Depletion: B cells are a type of immune cell that plays a role in the autoimmune response in MS. Monoclonal antibodies targeting CD20-positive B cells, such as ocrelizumab and rituximab, have been shown to reduce relapse rates and disability progression.
Neuroprotection and Remyelination: Some therapies focus on protecting nerve cells and promoting remyelination to repair damaged myelin. These approaches are still in experimental stages but show potential. Drugs like clemastine and opicinumab are being investigated for their ability to enhance remyelination.
Vitamin D Supplementation: There is evidence suggesting that vitamin D deficiency might play a role in MS development and progression. Some studies have shown that maintaining adequate levels of vitamin D through supplementation could have a positive impact on disease activity.
Anti-Inflammatory and Immunosuppressive Therapies: These treatments aim to control the immune response and reduce inflammation. Drugs like corticosteroids are often used to manage acute relapses and reduce inflammation in the central nervous system.
Myelin Repair Therapies: Researchers are exploring strategies to stimulate the repair and regrowth of damaged myelin. This includes promoting the differentiation of oligodendrocyte precursor cells into mature myelin-producing cells.
Personalized Medicine: With advancements in genetics and understanding of individual disease characteristics, personalized treatment plans are becoming more common. Tailoring therapies to the patient's specific disease profile could lead to better outcomes.
Mr. Jayesh Saini notes that, “The effectiveness of these therapies can vary from person to person, and treatment decisions should be made in consultation with a qualified healthcare provider. While significant progress has been made in the field of MS treatment, ongoing research is essential to continue improving therapies and potentially finding a cure in the future.”
#jayeshsaini #healthcare #LifeCareHospitals #Kenya #NHIF #NPS #TSC
0 notes
Text
PROMISING THERAPIES AND POTENTIAL CURES FOR MULTIPLE SCLEROSIS

Multiple sclerosis (MS) is a chronic autoimmune disease that affects the central nervous system,
specifically the brain and spinal cord. It is characterized by the immune system attacking the
protective covering of nerve fibers, known as myelin, leading to inflammation, demyelination
(loss of myelin), and a range of neurological symptoms. While there is currently no known cure
for MS, there are several promising therapies and potential treatments that aim to manage
symptoms, slow disease progression, and improve the quality of life for individuals with MS.
Disease-Modifying Therapies (DMTs): These treatments are designed to modify the course of
the disease by reducing the frequency and severity of relapses, slowing down disability
progression, and delaying the accumulation of new lesions in the brain and spinal cord. There are
several classes of DMTs, including interferons, glatiramer acetate, oral immunomodulators, and
monoclonal antibodies. Some examples of DMTs include:
• Interferons: These are proteins that help regulate the immune system. They can reduce
inflammation and the frequency of relapses. Examples include interferon beta-1a and
interferon beta-1b.
• Glatiramer Acetate: This is a synthetic protein that mimics myelin basic protein, helping
to divert the immune system's attack away from the actual myelin.
• Oral Immunomodulators: Drugs like fingolimod, siponimod, and teriflunomide modulate
immune responses and reduce inflammation. They are taken orally.
• Monoclonal Antibodies: Medications like ocrelizumab, alemtuzumab, and natalizumab
target specific immune cells or molecules involved in the disease process.
Stem Cell Therapy: Stem cell transplantation involves replacing the immune system with healthy
stem cells derived from the patient's own bone marrow or from a donor. This treatment aims to
"reset" the immune system and halt the autoimmune attack on myelin. Autologous
hematopoietic stem cell transplantation (aHSCT) has shown promising results in clinical trials for
aggressive forms of relapsing-remitting MS.
B-cell Depletion: B cells are a type of immune cell that plays a role in the autoimmune response
in MS. Monoclonal antibodies targeting CD20-positive B cells, such as ocrelizumab and rituximab,
have been shown to reduce relapse rates and disability progression.
Neuroprotection and Remyelination: Some therapies focus on protecting nerve cells and
promoting remyelination to repair damaged myelin. These approaches are still in experimental
stages but show potential. Drugs like clemastine and opicinumab are being investigated for their
ability to enhance remyelination.
Vitamin D Supplementation: There is evidence suggesting that vitamin D deficiency might play a
role in MS development and progression. Some studies have shown that maintaining adequate
levels of vitamin D through supplementation could have a positive impact on disease activity.
Anti-Inflammatory and Immunosuppressive Therapies: These treatments aim to control the
immune response and reduce inflammation. Drugs like corticosteroids are often used to manage
acute relapses and reduce inflammation in the central nervous system.
Myelin Repair Therapies: Researchers are exploring strategies to stimulate the repair and
regrowth of damaged myelin. This includes promoting the differentiation of oligodendrocyte
precursor cells into mature myelin-producing cells.
Personalized Medicine: With advancements in genetics and understanding of individual disease
characteristics, personalized treatment plans are becoming more common. Tailoring therapies
to the patient's specific disease profile could lead to better outcomes.
Mr. Jayesh Saini notesthat, “The effectiveness of these therapies can vary from person to person,
and treatment decisions should be made in consultation with a qualified healthcare provider.
While significant progress has been made in the field of MS treatment, ongoing research is
essential to continue improving therapies and potentially finding a cure in the future.”
0 notes
Text
Brand new CRISPR-Derived microRNA Realizing Mechanism Determined by Cas12a Self-Powered and also Moving Eliptical Transcription-Unleashed Real-Time crRNA Signing up.
The primary result calculated has been the actual renovation malfunction rate using prosthesis decline. Second endpoints had been capsular contracture along with artistic end result. Benefits: Absolutely no randomized managed tests ended up identified, and only a single future, nonrandomized, multicenter tryout was discovered. Reports exceeding 15 sufferers have been provided; 12 scientific studies were built with a total associated with 1853 people (715 irradiated and 1138 nonirradiated). Adjuvant radiotherapy resulted in a lot greater reconstruction failure rate within quick two-stage prosthetic busts renovation weighed against settings (Eighteen.6 % versus Several.1 percent, s smaller than 3.00001). Radiotherapy specifically increased your malfunction fee when given right after point One (expander) (Twenty nine.7 % vs . 5 percent, g smaller compared to 0.00001) and also point Two (long term embed) (Seven.7 % compared to One particular.5 percent, g Is equal to 2.0003). There is in addition more extreme capsular contractures with an substandard aesthetic increase the risk for irradiated patients. Finish: Nonrandomized studies suggest that adjuvant radiotherapy makes a greater risk involving reconstruction malfunction. Medical QUESTION/LEVEL Involving Proof: Restorative, 4.Gorassini MA, Norton JA, Nevett-Duchcherer L, Roy FD, Yang JF. Changes in locomotor muscle task soon after home treadmill trained in themes together with partial spine damage. J Neurophysiol Information and facts: 969-979, Last year. Initial posted 12 , 10, 08; doi: 15.1152/jn.91131.08. Extensive fitness treadmill machine education following unfinished vertebrae harm can enhance practical going for walks expertise. To look for the Teriflunomide changes in muscle mass activation designs which can be linked to advancements within strolling, we tested your electromyography (EMG) associated with achilles tendon within 18 people with incomplete spine damage through related going for walks conditions both both before and after training. Particular distinctions had been seen among topics in which sooner or later gained practical enhancements inside overground strolling (responders), in contrast to subject matter where fitness treadmill machine instruction ended up being unproductive (nonresponders). Although the two organizations designed a a lot more regular and much less clonic EMG structure about the fitness treadmill machine, it was exactly the tibialis anterior and also hamstring muscle tissue from the responders in which shown boosts in EMG account activation. Similarly, merely the responders demonstrated diminishes inside break open length along with cocontraction regarding proximal (hamstrings and quadriceps) muscles activity. Astonishingly, the particular proximal muscle tissue exercise within the responders, not like nonresponders, ended up being three- for you to fourfold more than that throughout uninjured manage subjects jogging in comparable rates and amount of bodyweight assist, indicating that the ability to change muscle account activation patterns following injury may predict light beer subject matter to further pay in response to motor instruction. To sum up, improves in the quantity and reduces inside the amount of EMG exercise of distinct muscle groups tend to be related to functional recovery regarding going for walks expertise right after treadmill machine lessons in topics that can alter muscles activity designs right after partial vertebrae injuries.
#Small Molecule Immuno-Oncology Compound Library#GSK-3 inhibitor#Brefeldin A#10058-F4#Nilotinib#FCCP#Ganciclovir#Sodium dichloroacetate#GSK343#S3I-201#D-Luciferin#BAY-876#KN-93#Ripasudil#Daunorubicin#GW9662#Ripretinib#Letrozole#Vincristine#N-Ethylmaleimide#Bemnifosbuvir#Glumetinib#Clopidogrel#Prednisone#Levonorgestrel#Fluconazole#Baloxavir#Abacavir#Alendronate#Teriflunomide
0 notes
Text
Multiple Sclerosis: Decrease in Relapses and Brain Lesions with Ublituximab, Targeting B Cells

MedicalResearch.com Interview with:

Prof. Steinman
Prof. Lawrence Steinman MD
Zimmermann Professor of Neurology & Neurological Sciences, and Pediatrics
Beckman Center for Molecular Medicine Stanford University
Stanford, CA
MedicalResearch.com: What is the background for this study? What are the main findings?
Response: We are publishing the results of two successful phase 3 trials in relapsing MS (multiple sclerosis).
We tested a glycoengineered antibody to B cells. The glycoengineered antibody are more potent in killing the target. They can be delivered more easily.
MedicalResearch.com: What should readers take away from your report?
Response: The reduction in relapses was better than any reported by other anti-CD20 antibodies. A barrier of 0.1 relapses per year had never been broken. Here in both trials the results showed fewer than 0.1 relapse per year, or in other words. less than one relpase in 10 years.
MedicalResearch.com: What recommendations do you have for future research as a results of this study?
Response: Since multiple sclerosis is triggered by an infection in B cells, combining antibodies that kill the infected cells with an anti-viral might be worthwhile to test.
MedicalResearch.com: Is there anything else you would like to add? Any disclosures?
Response: We also saw a statistically significant increase in disability improvement in these trials.
Citation:
Ublituximab versus Teriflunomide in Relapsing Multiple Sclerosis Lawrence Steinman, M.D., Edward Fox, M.D., Ph.D., Hans-Peter Hartung, M.D., Enrique Alvarez, M.D., Ph.D., Peiqing Qian, M.D., Sibyl Wray, M.D., Derrick Robertson, M.D., DeRen Huang, M.D., Ph.D., Krzysztof Selmaj, M.D., Ph.D., Daniel Wynn, M.D., Gary Cutter, Ph.D., Koby Mok, Ph.D., Yanzhi Hsu, Ph.D., Yihuan Xu, Ph.D., Michael S. Weiss, J.D., Jenna A. Bosco, B.S., Sean A. Power, B.S., Lily Lee, Ph.D., Hari P. Miskin, M.Sc., and Bruce A.C. Cree, M.D., Ph.D., for the ULTIMATE I and ULTIMATE II Investigators*
N Engl J Med 2022;387:704-14. DOI: 10.1056/NEJMoa2201904
https://www.nejm.org/doi/full/10.1056/NEJMoa2201904
The information on MedicalResearch.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition. Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
Read the full article
0 notes
Photo
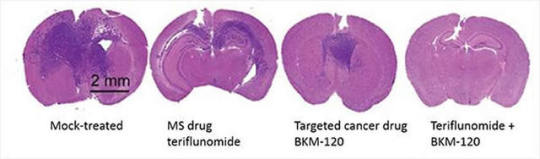
Adding MS drug to targeted cancer therapy may improve glioblastoma outcomes
Teriflunomide, a drug commonly used to treat multiple sclerosis, shows promise for the treatment of glioblastoma when coupled with targeted cancer therapies.
54 notes
·
View notes
Photo
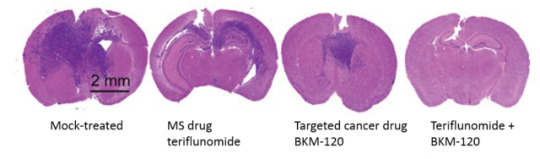
Adding MS Drug to Targeted Cancer Therapy May Improve Glioblastoma Outcomes
By reaching stem cells at the tumor’s root, multiple sclerosis drug teriflunomide, paired with targeted cancer therapy, markedly shrinks patient-derived glioblastomas grown in mice
Glioblastoma is an aggressive form of brain cancer that infiltrates surrounding brain tissue, making it extremely difficult to treat with surgery. Even when chemotherapy and radiation successfully destroy the bulk of a patient’s glioblastoma cells, they may not affect the cancer stem cells. This small population of tumor cells have the capacity to grow and multiply indefinitely, and can lead to tumor recurrence.
To study these tumors and test new therapies, researchers at University of California San Diego School of Medicine use mice that bear glioblastoma tumor samples donated by patients who underwent surgery. With this approach, they recently discovered that treatment with both a targeted cancer therapy and the multiple sclerosis (MS) drug teriflunomide halts glioblastoma stem cells, markedly shrinks tumors and improves mouse survival.
“We used to think we’d find a single magic bullet to treat everyone with glioblastoma,” said senior author Jeremy Rich, MD, professor of medicine at UC San Diego School of Medicine and director of neuro-oncology and director of the Brain Tumor Institute at UC San Diego Health. “But now we realize that we need to find out what drives each patient’s unique tumor, and tailor our treatments to each individual.”
Pictured above: In a patient-derived tumor model in mice, glioblastoma tumors (dark purple) shrink significantly when treated with both the MS drug teriflunomide and the targeted cancer drug BKM-120.
In recent years, the desire to personalize cancer therapies has led to the development of several targeted cancer therapies. These drugs work by inhibiting specific molecules that cancer cells rely on for growth and survival. For that reason, targeted therapies can work better and cause fewer side effects compared to traditional therapies, such as chemotherapy and radiation. Yet targeted therapies haven’t been as successful as the scientific community had hoped. According to Rich, that’s because it’s usually not enough to inhibit just one molecule or pathway driving tumor formation or survival — cancer cells will find a way to compensate.
“As scientists, we are often looking at small snapshot of what a cancer stem cell is doing,” said Rich, who is also a faculty member in the Sanford Consortium for Regenerative Medicine and Sanford Stem Cell Clinical Center at UC San Diego Health. “As a clinician, I also try to look at the bigger picture. I’m not looking for just one or two drugs to help my patients, because I think it’s going to take a whole personalized cocktail of many different drugs to really get the cancer cells on the run.”
26 notes
·
View notes
Text
Drugs that can be used to treat Multiple Sclerosis (MS), Part 2 of 3: Oral Disease-Modifying Agents (DMAs)
Dimethyl fumarate (Tecfidera)--DMF and its active metabolite, monomethyl fumarate (MMF), activate a cellular response pathway that responds to oxidative stress. Comes in 120 mg and 240 mg delayed-release capsules. Maintenance dose is 240 mg BID. Taken with food and/or aspirin to reduce flushing. May also cause GI upset and leukopenia/infection. There has been one case of fatal progressive multifocal leukoencephalopathy (PML) secondary to prolonged severe lymphopenia in Canada.
Fingolimod (Gilenya)--Sphingosine 1-Phosphate (S1P) Receptor Modulator that blocks WBCs' ability to leave lymph nodes, thereby decreased CNS inflammation. Dosage is 0.5 mg daily. May increase AST/ALT, especially in patients with hepatic impairment. Contraindicated in patients with most cardiac pathologies and/or CVA/TIA because it can cause HTN, AV block, bradycardia, and/or QT prolongation. There has been one reported case of (non-fatal) PML following the administration of Gilenya to a patient who had not previously received Tysabri (natalizumab), an MS drug associated with a higher risk of PML. Follow CBC, LFTs, EKG, ophthalmologic exam, VZV antibodies, and PFTs as indicated.
Teriflunomide (Aubagio)--A pyrimidine synthesis inhibitor that has antiproliferative and anti-inflammatory effects and may reduce the number of activated lymphocytes in the CNS. It is given 7 mg or 14 mg PO daily. It has the potential for significant hepatotoxicity, so monitor LFTs. It is teratogenic. May cause alopecia.
4 notes
·
View notes
Photo
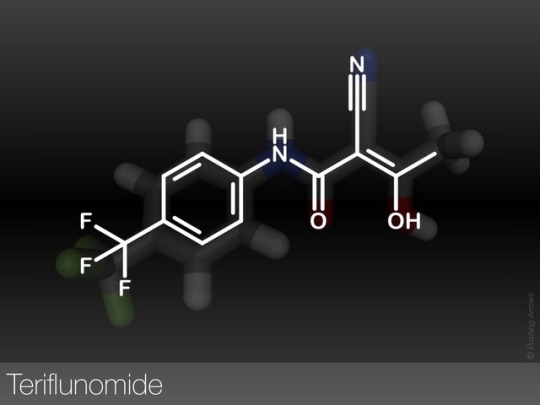
Teriflunomide - an inhibitor of the enzyme dihydroorotate dehydrogenase hence in turn inhibiting pyrimidine de novo synthesis. It is used for the treatment of multiple sclerosis (MS) by inhibiting rapidly dividing cells like activated T cells which are thought to drive the MS process.
It is also the main active in vivo metabolite of leflunomide by the opening of its isoxazole ring.
#teriflunomide#inhibitor#enzyme#dihydroorotate dehydrogenase#pyrimidine de novo synthesis#multiple sclerosis#MS#T cell#leflunomide#isoxazole
0 notes
Text
Teriflunomide Tempers Lesions in Kids With Multiple Sclerosis
Teriflunomide Tempers Lesions in Kids With Multiple Sclerosis
In TERIKIDS, 109 children were randomized to receive teriflunomide and 57 were randomized to receive placebo for up to 96 weeks.
Open-label extension phase entry was possible before the end of the double-blind period for patients who experienced a relapse or demonstrated high disease activity on MRI imaging tests.
More patients in the placebo group entered the open-label extension phase than…
View On WordPress
0 notes
Text
PROMISING THERAPIES AND POTENTIAL CURES FOR MULTIPLE SCLEROSIS

Multiple sclerosis (MS) is a chronic autoimmune disease that affects the central nervous system,
specifically the brain and spinal cord. It is characterized by the immune system attacking the
protective covering of nerve fibers, known as myelin, leading to inflammation, demyelination
(loss of myelin), and a range of neurological symptoms. While there is currently no known cure
for MS, there are several promising therapies and potential treatments that aim to manage
symptoms, slow disease progression, and improve the quality of life for individuals with MS.
Disease-Modifying Therapies (DMTs): These treatments are designed to modify the course of
the disease by reducing the frequency and severity of relapses, slowing down disability
progression, and delaying the accumulation of new lesions in the brain and spinal cord. There are
several classes of DMTs, including interferons, glatiramer acetate, oral immunomodulators, and
monoclonal antibodies. Some examples of DMTs include:
• Interferons: These are proteins that help regulate the immune system. They can reduce
inflammation and the frequency of relapses. Examples include interferon beta-1a and
interferon beta-1b.
• Glatiramer Acetate: This is a synthetic protein that mimics myelin basic protein, helping
to divert the immune system's attack away from the actual myelin.
• Oral Immunomodulators: Drugs like fingolimod, siponimod, and teriflunomide modulate
immune responses and reduce inflammation. They are taken orally.
• Monoclonal Antibodies: Medications like ocrelizumab, alemtuzumab, and natalizumab
target specific immune cells or molecules involved in the disease process.
Stem Cell Therapy: Stem cell transplantation involves replacing the immune system with healthy
stem cells derived from the patient's own bone marrow or from a donor. This treatment aims to
"reset" the immune system and halt the autoimmune attack on myelin. Autologous
hematopoietic stem cell transplantation (aHSCT) has shown promising results in clinical trials for
aggressive forms of relapsing-remitting MS.
B-cell Depletion: B cells are a type of immune cell that plays a role in the autoimmune response
in MS. Monoclonal antibodies targeting CD20-positive B cells, such as ocrelizumab and rituximab,
have been shown to reduce relapse rates and disability progression.
Neuroprotection and Remyelination: Some therapies focus on protecting nerve cells and
promoting remyelination to repair damaged myelin. These approaches are still in experimental
stages but show potential. Drugs like clemastine and opicinumab are being investigated for their
ability to enhance remyelination.
Vitamin D Supplementation: There is evidence suggesting that vitamin D deficiency might play a
role in MS development and progression. Some studies have shown that maintaining adequate
levels of vitamin D through supplementation could have a positive impact on disease activityAnti-Inflammatory and Immunosuppressive Therapies: These treatments aim to control the
immune response and reduce inflammation. Drugs like corticosteroids are often used to manage
acute relapses and reduce inflammation in the central nervous system.
Myelin Repair Therapies: Researchers are exploring strategies to stimulate the repair and
regrowth of damaged myelin. This includes promoting the differentiation of oligodendrocyte
precursor cells into mature myelin-producing cells.
Personalized Medicine: With advancements in genetics and understanding of individual disease
characteristics, personalized treatment plans are becoming more common. Tailoring therapies
to the patient's specific disease profile could lead to better outcomes.
Mr. Jayesh Saini notesthat, “The effectiveness of these therapies can vary from person to person,
and treatment decisions should be made in consultation with a qualified healthcare provider.
While significant progress has been made in the field of MS treatment, ongoing research is
essential to continue improving therapies and potentially finding a cure in the future.”
0 notes
Text
Tweeted
#MS_Selfie_Case_Study - A patient who is NEDA-3 on fingolimod and is considering derisking his immunosuppression. Does he go lower and switch to teriflunomide or higher and choose an IRT? Will he have regrets if does nothing? What an interesting dilemma! https://t.co/spb1ZcZtSs https://t.co/COwVdQKJZ8
— Gavin Giovannoni (@GavinGiovannoni) Oct 21, 2021
0 notes
Text
NICE says yes to Novartis’ multiple sclerosis therapy Kesimpta
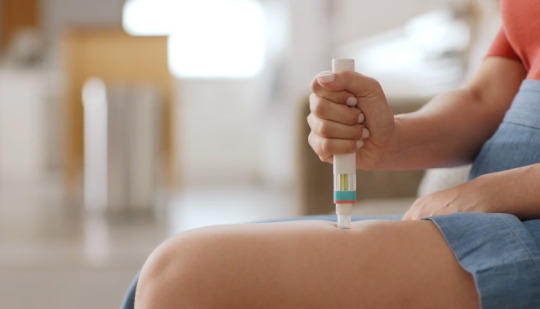
Novartis has secured backing from NICE for its relapsing multiple sclerosis (RMS) therapy Kesimpta in the UK, just two weeks after the drug was approved by the national drugs regulator.
The cost-effectiveness agency has said that anti-CD20 antibody Kesimpta (ofatumumab) can be prescribed via the NHS in England and Wales as a treatment for adults with RMS with active disease, as either a first-line therapy or after alternative drugs have been tried.
According to Novartis, Kesimpta is the first targeted B cell therapy for RMS that can be self-administered at home once a month using an autoinjector, avoiding the need for patients to travel to hospital during the pandemic, and easing the pressure on clinicians.
NICE’s final appraisal document for Kesimpta will be followed by technology appraisal guidance (TAG) that will pave the way for NHS prescribing in England and Wales. The Scottish Medicines Consortium is expected to publish its final advice on the drug later this year.
Kesimpta was one of the first medicines to be review under the new Medicines and Healthcare products Regulatory Agency (MHRA) authorisation process following the UK’s departure from the EU at the start of the year.
It was approved on the strength of two phase 3 studies (ASCLEPIOS 1 and 2) which showed that Kesimpta was more effective than Sanofi’s Aubagio (teriflunomide) in reducing annualised relapse rates and disability progression.
Nearly nine out of ten patients treated with Kesimpta saw no evidence of disease activity in their second year of treatment with the drug.
Relapsing and remitting forms of MS account for around 85% of all patients at diagnosis, although some go on to develop more progressive forms. There are around 130,000 people with MS in the UK, with 7,000 new diagnoses a year.
“People living with MS need access to a range of different treatment options so they can work with their doctor to find the one that’s best for them,” said David Martin, chief executive of the Multiple Sclerosis Trust.
“Without the need for regular hospital visits, even those who live a long way from a specialist centre will be able to access treatment that could reduce disease activity and help them continue to do the things that matter most to them,” he added.
Analyst Philippa Salter of GlobalData recently predicted that Kesimpta’s convenience will make it a sought-after option for RMS patients, driving global sales to $3.3 billion by 2028.
However, she reckons it will still trail Roche’s first-to-market anti-CD20 drug Ocrevus (ocrelizumab), even though that drug needs to be administered by intravenous infusion. While it requires a clinic visit, Ocrevus only has to be administered twice a year.
GlobalData thinks Ocrevus will remain the most widely-used option, hitting $7.6 billion in sales in 2028. It was backed by NICE for NHS use in July 2018.
Kesimpta started life as an oncology drug and was first developed by Genmab and licensed to GlaxoSmithKline who marketed under the brand name Arzerra to treat chronic lymphocytic leukaemia. Novartis took ownership of the drug in 2015.
The post NICE says yes to Novartis’ multiple sclerosis therapy Kesimpta appeared first on .
from https://pharmaphorum.com/news/nice-says-yes-to-novartis-multiple-sclerosis-therapy-kesimpta/
0 notes
Text
Ponesimod bests teriflunomide in lowering relapse rates of multiple sclerosis; JAMA
The research has been published in JAMA Neurology. | "To our knowledge, the Oral Ponesimod Versus Teriflunomide In Relapsing Multiple Sclerosis ( ...
from Google Alert - neurological https://ift.tt/2PRaHcc
0 notes
Text
Multiple Sclerosis Therapeutics Market with Covid-19 Pandemic Analysis, Growth Rate, New Trend Analysis Forecast To 2027

Industry Overview of the Multiple Sclerosis Therapeutics Market 2020
The research report titled Global Multiple Sclerosis Therapeutics Market is an all-inclusive document containing crucial data pertaining to sales and revenue of the Multiple Sclerosis Therapeutics industry. The evaluations are done on the basis of the analysis of leading market segments such as types, applications, regions, technological advancements, and dominant players of the global Multiple Sclerosis Therapeutics industry. The report provides historical data (2017-2018) of the industry and provides valuable forecast information for the period of 2020-2027. The report is formulated with the analysis of current and emerging trends based on the impact of the COVID-19 pandemic. The pandemic has changed the dynamics of the market and has drastically shaken up the economic scenario of the world. The report covers an impact analysis of the COVID-19 pandemic and offers a futuristic outlook of a post COVID scenario. It also covers trends, demands, and growth rates impacted by the ongoing crisis.
The forecast estimation states the global Multiple Sclerosis Therapeutics market is expected to dominate the economic sphere of the world with significant growth in the coming years. The growth is boosted by a change in demand patterns, rapidly developing infrastructure, technological advancements, and product advancements. The current and emerging trends are expected to shape up the industry and help in gaining a strong foothold in the global market to contribute to the revenue generation.
The Global Multiple Sclerosis Therapeutics market is further analyzed on the basis of key companies operating in the business sphere and major geographical regions where the market has a substantial size and growth rate.
Prominent Players: Biogen Idec, Teva Pharmaceuticals, Bayer Healthcare, Pfizer, Sanofi-Aventis, Merck, and Novartis, AbbVie Inc., Acorda Therapeutics Inc.
Product Type (Revenue, USD Million; 2016–2027)
Ponesimod
Glatopa
Copaxone
Avonex
Fingolimod
Teriflunomide
Others
Cladribine
Others
Prednisone
Methylprednisolone
Betaseron
Others
Mode of Administration Type (Revenue, USD Million; 2016–2027)
Injectable
Intravenous
Oral
Distribution Channel (Revenue, USD Million; 2016–2027)
Healthcare Providers
Online Pharmacies
Medical Clinics
Others
Zonal Partition of the Market: North America, Latin America, Europe, Asia-Pacific, and the Middle East & Africa.
The report covers extensive analysis of market segments that are anticipated to lead by the end of the forecast period (2020-2027). The report puts a special emphasis on the upstream raw materials, downstream buyers, industrial chain analysis, technological and product advancements, and production and manufacturing capacities of the Multiple Sclerosis Therapeutics market. Moreover, the report provides an in-depth analysis of the core segments of the market by analysis of the applications, types, consumption patterns, market drivers and restraints, and challenges to be faced in the market.
The research study focuses on the emerging development patterns and manufacturing processes anticipated to boost the growth of the market. It also includes extensive profiles of prominent contenders of the industry and provides a complete analysis inclusive of their market share, market size, production capacity, sales and distribution network, import/export activity, and product portfolios.
To read more about the report @ https://www.reportsanddata.com/report-detail/multiple-sclerosis-therapeutics-market
Major objectives of the Global Multiple Sclerosis Therapeutics Report:
· Analysis and forecast estimation of the Global Multiple Sclerosis Therapeutics Market based on the market segmentation into types, applications, and regions
· Analysis of micro and macroeconomic factors affecting the global Multiple Sclerosis Therapeutics market
· Valuable insight into the major drivers, limitations, opportunities, and challenges faced by the global Multiple Sclerosis Therapeutics market and its players
· In-depth analysis of the prominent contenders along with their business strategies and expansion plans
· Strategic recommendations to the established companies as well as new entrants to assist in the formulation of investment plans
· Comprehensive analysis of the competitive landscape of the Multiple Sclerosis Therapeutics industry
To summarize, the report provides a better understanding to the reader about the Multiple Sclerosis Therapeutics industry by offering a detailed explanation of the competitive landscape, industry environment, market projections, growth driving and restraining factors, limitations, entry barriers, and opportunities. The report also covers the regulatory framework, investment opportunities, and other growth driving factors. The report allows the reader to gather insightful information about each segment of the market and provides a historical, present, and prospective outlook of the market.
Thank you for reading our report. To know more about the report or for any queries regarding customization, please connect with us. Our team will provide excellent assistance and make sure the report is tailored to meet your requirements.
Related Reports –
SB Wire – http://www.sbwire.com/press-releases/multiple-sclerosis-therapeutics-market-to-reach-usd-2669-billion-by-2027-teva-pharmaceuticals-bayer-healthcare-pfizer-sanofi-aventis-etc-1300643.htm
Digital Journal – http://www.digitaljournal.com/pr/4781272
0 notes