#FDA Approves First Breath Test for COVID
Note
Tucker Carlson cherry-picked through the 1/6 footage to try and portray this as a "normal" day at the Capitol when it came to tours. One Republican said it best. It they wanted to tour the Capitol they should have gotten in line like normal people do when they want a tour. People broke into the Capitol. People brutalized Capitol Guards and that's a fact lost on Trumptards. Congressman were hiding in the House Chambers as these rioters tried to break in. Why can't you see that for what it is. As to the COVID "hoax", well, I've known some nurses and doctors who have a differing opinion than you do since they've been on the front lines of it. It's sad that some like yourself are in denial.
Tucker “cherry-picked”? That’s ingenious from someone who (as in you), obviously believe all the propaganda bs the likes of CNN spewed on the nation. My take would be this….if they were truly rioting, and IF they wanted congress member dead, they would be dead. Why were they hiding? Why didn’t they come out and take control of the situation? If middle America wanted to stage a take over, it would have happened. There is plenty of gun power among the average citizen….and this is precisely why Americans should never surrender their right to bear arms. In all fairness, if there is nothing to hide, then why is congress having to demand the footage be released?
One thing I’ve never done is call anyone a Bidentard. To lower yourself to the point that would infer someone is a “retard” is not only juvenile but extremely insulting. People on your side of things love to name call. Whether it’s a “Trumptard”, “xenophobic”, “racist”. I simply see you as someone with a differing view. We don’t all have to agree or believe the same thing. But at least I treat people with civility.
As for the covid “hoax”? I never said it was a hoax. I’ve been a nurse for 20+ years. I worked almost 15 years in a Level I trauma, magnet certified pediatric intensive care unit. I have seen first hand what flu and rsv can do to a child’s body. I NEVER said covid was a hoax, I said it’s a VIRUS, just like the flu and rsv. Let me break it down for you. Viruses will kill people. Every year seemingly healthy children and adults die from viruses. My problem is the fact that the government lied to its citizens. The vaccine is not effective. It hasn’t been properly tested. If you don’t know what is entailed in a vaccine being approved you should educate yourself. It takes YEARS of trials and studies before being approved by the FDA. so ask yourself who stood to profit from a vaccine being mass produced and rolled out so quickly. Do not EVER make the mistake of saying i dont know what the front line is like. I’ve seen the front line first hand. That’s a stupid title to give to people who have gone to work for years and taken care of people with a multitude of infectious diseases and never needed any appreciation for it. Every day I went to work and put on an isolation gown and mask. I’ve seen a baby with RSV struggle to breath. I’ve seen parents filled with fear because their child was losing every extremity to meningitis. You don’t know the first thing about healthcare…but we go to work every day and never need praise for what we do. We just do the job.
48 notes
·
View notes
Text
FDA Approves at-home Duel COVID and Flu Test
The Food and Drug Administration (FDA) has approved the first at-home test that can be used to determine whether a person has the flu or COVID-19.
Duel COVID and Flu Test
Testing for the flu previously required a visit to a doctor’s office or urgent care. The test will be sold over the counter, and it can detect both Influenza A and Influenza B, as well as the SARS-CoV-2 virus (the virus that causes COVID-19).
The single-use test works by using a nasal swab that is placed in the test unit. Results are provided within 30 minutes.
The test correctly identified 99.3 percent of samples negative for Influenza A while correctly identifying 90.1 percent of samples positive for the virus. The test also properly identified 99.9 percent of samples negative for Influenza B in a small sample size.
The test correctly identified 100 percent of tests negative for COVID-19 and 88.3 percent of COVID-19 samples, according to the FDA.
Studies show that an at-home test can be less accurate than a lab test because users might not get enough of a sample for a valid result. Health care providers also may have more sensitive tests.
Signs and Symptoms of COVID-19 and the Flu
COVID-19 and the flu have many symptoms in common, including:
Cough
Fever
Headache
Muscle aches
Nausea or vomiting
Runny or stuffy nose
Shortness of breath or difficulty breathing
Tiredness
Symptoms of the two appear at different times, however. COVID-19 symptoms appear two to 14 days after exposure. Flu symptoms appear about one to four days after exposure.
What IAA has to Say
Insurance Administrator of America wants to keep you up to date on the world of health. Stay tuned to this blog to learn more. Remember, with IAA one call does it all.
2 notes
·
View notes
Text
WHAT SHOULD YOU KNOW ABOUT RESPIRATORS, N95- KN95
N95 and KN95: Know the Appropriate Differences
The use of face masks is recommended as part of the health measures to take during this pandemic. On the other hand, their use is inextricably related to social and cultural practices and has come to have a variety of personal and societal implications. In order to prevent COVID-19 transmission and save lives, masks should be used as part of a comprehensive effort.
It is necessary to be safe while taking all the precautions and guidelines when COVID-19 is spreading in the community, including physical separation, wearing a mask, keeping rooms properly aired, washing hands, washing hands properly, and coughing into a tissue. Make it a natural part of social interactions to wear a mask. Masks must be used, kept, cleaned, and disposed of appropriately in order to be as effective as possible.
Essential things to keep in mind while using a mask
-Getting your hands clean is an important factor while using a mask, whether you’re putting it on or removing it from your face.
-It is also important to make sure that your nose, chin, and mouth are covered properly.
-When you remove a mask, put it in a clean trash container and wash it or throw it away if it’s a fabric mask.
-Masks with valves should not be worn.
About N95
In the medical field and the construction business, the N95 respirator is regarded as the gold standard of face protection. The borders of these face coverings are meant to fit securely on your face, unlike surgical masks.
N95 respirators are defined by the US Food and Drug Administration (FDA) as a “protective device designed to create a very tight face fit and extremely effective airborne particle filtering.” However, the Centers for Disease Control and Prevention (CDC) continue to advise against their use by the general population. But that’s not because the masks are useless, but to save resources for healthcare personnel and medical first responders.
About KN95
Despite the fact that KN95s and N95s are quite similar, only the latter is allowed to be used in medical settings in the United States. The reason is simple: N95s are the American standard for these tight-fitting filtration devices, whereas KN95s are the Chinese standard. Both are capable of filtering out 95% of every minute particle.
The CDC has approved the use of KN95 masks as a viable alternative to N95 masks due to a shortage of personal protective equipment (PPE) in the United States at the outset of the pandemic. Unfortunately, a few hospitals and other KN95 users have noticed notable variances in quality.
N95 AND KN95
Cloth masks aren’t enough to prevent users from breathing virus-carrying particles.
N95 and KN95 respirators shield users from particles, including the virus that causes COVID-19, according to the CDC. However, they also protect others from droplets and particles coming out of the one wearing the mask.
Certain breathing apparatuses have been tested to ensure that they fulfill international health standards and are labeled to inform consumers of their compliance. According to the CDC, KN95s are the most readily accessible respirators that fulfill international criteria.
On the other hand, others take a step further and adhere to a specific United States standard, which includes a quality criterion, as set forth by the National Institute for Occupational Safety and Health (NIOSH). N95 respirators are among these.
The CDC advises healthcare personnel to prioritize N95s with a specific “surgical” designation.
The differences between N95 and KN95
It is essential to know the differences between the two masks. Some of the key distinctions are being described below:
The Chinese government demands a unique mask fit test on people with an 8 percent leakage rate to be approved as a KN95 mask.
Manufacturers are not required to conduct fit testing under the N95 mask standard.
This isn’t to say that fit tests aren’t beneficial. Many hospitals and companies require mask fit testing of their staff. However, those stated to be the needs and requirements of the companies, and they are not for the US NIOSH certification on the mask.
Pressure drop during inhalation is slightly more stringent with N95 masks. As a result, they must be a little more ventilated than the KN95 masks.
Don’t stress or worry whenever you face a bit of difficulty in breathing through the mask, as dying from suffocation or oxygen starvation is very unlikely while wearing a mask.
N95s feature somewhat tougher pressure drop restrictions while exhaling, which could improve breathability.
How can you know if your mask is genuine?
Some counterfeit respirators are promoted and sold as NIOSH-approved. According to the CDC, this means they might not be able to provide enough respiratory protection.
NIOSH-approved respirators such as N95 masks will include an approval label on or inside the packaging. In addition, an abridged approval will be printed on the respirator.
What should you know about respirators certified by the National Institute of Occupational Safety and Health (NIOSH)?
When worn regularly and correctly, they give the maximum amount of protection from particles, including the virus that causes COVID-19. They also keep your respiratory secretions and particulates contained, so you don’t expose others. When correctly adjusted, they form a tight seal around your face. Because not all respirators fit the same, it’s critical to choose one that effectively fits your face and seals. NIOSH-approved respirators are tested to a US standard that includes a quality criterion.
When NIOSH approves them, it is suitable and efficient to use. They filter at least 95% of particles in the air after making sure they’re fitted suitably.
Conclusion
N95 and KN95 masks are nearly identical for the average individual. If you’re not a medical professional, one of these options should suffice. Thus it is necessary to know the important facts about each of them and use them to protect ourselves from the viruses as such COVID-19
0 notes
Text
Catherine Falardeau - phaware® interview 370
Scleroderma patient, Catherine Falardeau, discusses her road to diagnosis and how a right heart catheterization, led to also being diagnosed with PAH, ILD, Raynaud's, and Pulmonary Fibrosis. Catherine recently participated in a 108 week clinical trial for the use of TYVASO® in PAH/ILD patients. The trial lasted into Covid, and received FDA approval.
My name is Catherine Falardeau and I have had pulmonary hypertension since 2017 when I got diagnosed. I was working as a contractor here in Florida, and I started experiencing shortness of breath. It got to the point where I could barely go to the mailbox anymore. I had gone to the doctors and they noticed some spots on my face. So they gave me an ANA test to test for autoimmune diseases. In 2015, they told me I had scleroderma. I didn't know what the heck that was. The scleroderma affects the collagen both externally as well as internally. Due to the fact that I started experiencing such shortness of breath, I went to a pulmonologist. They gave me pulmonary function tests, which I did horrible on. They found out I only had 52% lung capacity.
They sent me to a cardiologist. He did all kinds of testing. When I had my right heart catheterization in 2018, that's when he found out that I had not only pulmonary hypertension, but also interstitial lung disease along with GERD and Raynaud's and all the things that go along with that. At first, I was just freaked out. I didn't know what to do.
He brought up the fact that he was conducting a clinical trial with people that had PAH as well as ILD. The first part of the trial was you didn't know if you were going to get the actual drug or a sugar pill. After three months or so of the ending of part one of that study, I signed on for part two. Part two was you definitely were getting the drug and I could almost immediately start to feel a big difference. I wasn't quite as tired. I wasn't quite as short of breath. I still have coughing spells, but it's usually right after I take a treatment. That's how I found out I had the pulmonary hypertension.
I was depressed for about a year. I sought therapy during that time. Then I started making small changes in my life. For one, I had to become a sudden caretaker for my mom up in Massachusetts. I spent a total of three months in 2019 up in Massachusetts, not only taking care of her, but I kept getting sick in the process from the travel going back and forth and it was really hard. We finally convinced her to move down to Florida to live here in assisted living.
I'm still trying to battle along with the clinical trial and appointments and then COVID came along. It was 108-week study, and we were nearing around, I think it was 90 something when the COVID kicked in. Because of the isolation we had to carefully get to the point where I could get to the final phase, which was turning in all the equipment and doing the final tests, which I finally was able to do in September. The study officially ended in September of 2020. It was the use of TYVASO® with patients that have not only pulmonary hypertension, but also interstitial lung disease. The FDA did reach approval, and I understand that's moving forward.
I also participated in the genomic testing, where they will use my blood samples for future research and development of new medications. I think it's extremely important, not only for one as a person, but it gives you the ability to be able to participate in forums like this. I like to do this a lot to spread awareness. I participate in Rare Patient Voice as well, and I'm a PH mentor, so I help people that have questions. For example, my last one was, how do I apply for social security disability? I said, well, first you need to hire a lawyer is the best part, the best way to go around that. Don't try to go to SSDI by yourself. Chances are you'll be rejected.
If you want to go to therapy, I would recommend therapy for people. My first thought is having a positive attitude. What I try to do is I wake up and I write down three things that I appreciate about the day. You could do it at the end of the day, and just say one of the things, "Hey, I woke up, it's another day, it's a positive. I can go live my life." I feel it's important to journal your thoughts. I have a whole online journal. I find it important to keep your mind busy. If I'm not doing this, I'm doing volunteer work for a dachshund rescue group in Florida. I post the stories of the dogs and how they came to the rescue and how they could make your home better just by having a dachshund. They're loyal. It helps my mind because I do this using WordPress, which I taught myself. I was an IT person for over 30 years. I feel that those kind of projects are important to keep your mind off anything negative and stay focused on that you're living your life every day.
I don't feel like it's a death sentence and it shouldn't be looked at that way. I feel it's important for people not to get on Google and start searching for the years that you have left or whatever. It's important to be proactive, especially since I just turned 60 about making arrangements and things like that. I feel lucky that I also have my husband who's gone through all this journey.
Now I'm at the point as long as I take my medication and I go to my doctor's visits, I highly recommend that when the doctor gives you a drug, don't give up on it because it makes you cough or it has unpleasant side effects. I went through that when I first went on the medication for scleroderma, which is mycophenolate, which is pretty common among autoimmune diseases -- lupus and scleroderma and the like.
It's important to keep all your doctor's appointments. Write down all your concerns. For example, if the medications, make a list of side effects. And I did this after my COVID booster. I wrote down all the side effects I had, in case I get involved in a COVID study, that I could tell someone here's what happened to me after I received my booster. You need to write it down as it happens. Don't wait to get to the doctor's office and then they may ask you "How are you doing on the medication?" "Well, I had side effects." And then you may not remember each and every one. It's important to write everything down or type it if you're like me, and you can't read your own writing.
It's important to type everything up and make sure you keep good medical records. I have my medical records ordered by specialty and not by doctor, because I've found that I've changed primary care doctors three times and so it's best to just put PCP and put it in a filing cabinet. Or if you have an electronic method that works, you can always go to the PHA Association. On their website, they probably have something about it.
I know I participated in a webinar about the best medical records keeping practices. I also contributed by telling the audience how I do mine. Everybody might do theirs differently. Also keep a small folder of your doctor's names, your condition names, and all the medications that you take and keep it with you. You leave it in your car, if you have one, to just take it with you wherever you go, so that if something were to happen to you, a policeman or whoever jumps in can look through your stuff and flag if you're unconscious and can't speak for yourself. I think that's a very important thing. That would be my advice and stay positive.
My name is Catherine Falardeau and I'm aware that I'm rare.
Learn more about pulmonary hypertension trials at www.phaware.global/clinicaltrials. Never miss an episode with the phaware® podcast app. Follow us @phaware on Facebook, Twitter, Instagram, YouTube & Linkedin Engage for a cure: www.phaware.global/donate #phaware #ClinicalTrials #scleroderma
Listen and View more on the official phaware™ podcast site
0 notes
Link
BY ANITA SIRCARAUG. 17, 2021 9:28 AM PT
My patient sat at the edge of his bed gasping for air while he tried to tell me his story, pausing to catch his breath after each word. The plastic tubes delivering oxygen through his nose hardly seemed adequate to stop his chest from heaving. He looked exhausted.
He had tested positive for the coronavirus 10 days ago. He was under 50, mildly hypertensive but otherwise in good health. Eight days earlier he started coughing and having severe fatigue. His doctor started him on antibiotics. It did not work.
Fearing his symptoms were worsening, he started taking some hydroxychloroquine he had found on the internet. It did not work.
He was now experiencing shortness of breath while doing routine daily activities such as walking from his bedroom to the bathroom or putting on his shoes. He was a shell of his former self. He eventually made his way to a facility where he could receive monoclonal antibodies, a lab-produced transfusion that substitutes for the body’s own antibodies. It did not work.
He finally ended up in the ER with dangerously low oxygen levels, exceedingly high inflammatory markers and patchy areas of infection all over his lungs. Nothing had helped. He was getting worse. He could not breathe. His wife and two young children were at home, all infected with COVID. He and his wife had decided not to get vaccinated.
Last year, a case like this would have flattened me. I would have wrestled with the sadness and how unfair life was. Battled with the angst of how unlucky he was. This year, I struggled to find sympathy. It was August 2021, not 2020. The vaccine had been widely available for months in the U.S., free to anyone who wanted it, even offered in drugstores and supermarkets. Cutting-edge, revolutionary, mind-blowing, lifesaving vaccines were available where people shopped for groceries, and they still didn’t want them.
Outside his hospital door, I took a deep breath — battening down my anger and frustration — and went in. I had been working the COVID units for 17 months straight, all day, every day. I had cared for hundreds of COVID patients. We all had, without being able to take breaks long enough to help us recover from this unending ordeal. Compassion fatigue was setting in. For those of us who hadn’t left after the hardest year of our professional lives, even hope was now in short supply.
Shouting through my N95 mask and the noise of the HEPA filter, I introduced myself. I calmly asked him why he decided not to get vaccinated.
“Well, I’m not an anti-vaxxer or anything. I was just waiting for the FDA to approve the vaccine first. I didn’t want to take anything experimental. I didn’t want to be the government’s guinea pig, and I don’t trust that it’s safe,” he said.
“Well,” I said, “I can pretty much guarantee we would have never met had you gotten vaccinated because you would have never been hospitalized. All of our COVID units are full and every single patient in them is unvaccinated. Numbers don’t lie. The vaccines work.”
This was a common excuse people gave for not getting vaccinated, fearing the vaccine because the Food and Drug Administration had only granted it emergency-use authorization so far, not permanent approval. Yet the treatments he had turned to, antibiotics, monoclonal antibodies and hydroxychloroquine were considered experimental, with mixed evidence to support their use.
The only proven lifesaver we’ve had in this pandemic is a vaccine that many people don’t want. A vaccine we give away to other countries because supply overwhelms demand in the U.S. A vaccine people in other countries stand in line for hours to receive, if they can get it at all.
“Well,” I said, “I am going to treat you with, remdesivir, which only recently received FDA approval.” I explained that it had been under an EUA for most of last year and had not been studied or administered as widely as COVID-19 vaccines. That more than 353 million doses of COVID-19 vaccine had been administered in the U.S. along with more than 4.7 billion doses worldwide without any overwhelming, catastrophic side effects. “Not nearly as many doses of remdesivir have been given or studied in people and its long-term side effects are still unknown,” I said. “Do you still want me to give it to you?”
“Yes” he responded, “Whatever it takes to save my life.”
It did not work.
My patient died nine days later from a fatal stroke. We, the care team, reconciled this loss by telling ourselves: He made a personal choice not to get vaccinated, not to protect himself or his family. We did everything we could with what we had to save him. This year, this tragedy, this unnecessary, entirely preventable loss, was on him.
The burden of this pandemic now rests on the shoulders of the unvaccinated. On those who are eligible to get vaccinated, but choose not to, a decision they defend by declaring, “vaccination is a deeply personal choice.” But perhaps never in history has anyone’s personal choice impacted the world as a whole as it does right now. When hundreds and thousands of people continue to die, when the most vulnerable members of society, our children, cannot be vaccinated — the luxury of choice ceases to exist.
If you believe the pandemic is almost over and you can ride it out, without getting vaccinated, you could not be more wrong. This virus will find you.
If you believe I’ll just wait until the FDA approves the vaccine first, you may not live to see the day.
If you believe if I get infected I’ll just go to the hospital and get treated, there is no guarantee we can save your life, nor even a promise we’ll have a bed for you.
If you believe I’m pregnant and I don’t want the vaccine to affect me, my baby or my future fertility, it matters little if you’re not alive to see your newborn.
If you believe I won’t get my children vaccinated because I don’t know what the long-term effects will be, it matters little if they don’t live long enough for you to find out.
If you believe I’ll just let everyone else get vaccinated around me so I don’t have to, there are 93 million eligible, unvaccinated people in the “herd” who think the same way you do and are getting in the way of ending this pandemic.
If you believe vaccinated people are getting infected anyway so what’s the point?, the vaccine was built to prevent hospitalizations and deaths from severe illness. Instead of fatal pneumonia, those with breakthrough infections have a short, bad cold, so the vaccine has already proved itself. The vaccinated are not dying from COVID-19.
SARS-CoV-2, the virus that causes COVID-19, has mutated countless times during this pandemic, adapting to survive. Stacked up against a human race that has resisted change every step of the way — including wearing masks, social distancing, quarantining and now refusing lifesaving vaccines — it is easy to see who will win this war if human behavior fails to change quickly.
The most effective thing you can do to protect yourself, your loved ones and the world, is to GET VACCINATED.
And it will work.
Anita Sircar is an infectious disease physician and clinical instructor of health sciences at the UCLA School of Medicine.
#op-ed#Anita Sircar#vaccination#global pandemic#COVID-19#LA Times#the plague#get vaccinated#science#medicine
8 notes
·
View notes
Text
FIP: Feline Infectious Peritonitis

Sprinkles contemplates some birds. We’re re-doing the catios right now, so they’re all closed off. I think she’s looking forward to being outdoors again.
I’m putting a cut here because this is a LENGTHY READ and, in case of further developments, I’d like to be able to easily update this article.
In October 2019, she was diagnosed with ocular FIP (Feline infectious peritonitis), which is a mutation of feline coronavirus (FCoV, which is very distinct from SARS-CoV-2, the virus that causes COVID-19). FCoV is ubiquitous in the cat population: almost every cat has it or is exposed to various strains of it. Most cats get over it just fine with only mild diarrhea. In a small percentage of cats (we’re uncertain on the percentage, which I’ll get into later, but it’s theoretically somewhere between 5-10%), it goes fuckwhack apeshit and mutates into FIP.
We don’t know why it spontaneously mutates. There seems to be a genetic component to it. It’s believed to be more common in purebred cats, but we’re really not sure--- since FIP is a diagnosis by exclusion, there often is a hefty vet bill attached to the diagnosis and a person who can afford to buy a purebred cat from a cattery is more likely to be able to afford that bill. It MAY be triggered by stress. It’s much more common in younger cats, often appearing in kittens ranging from 4 months to 4 years. This doesn’t mean older cats are safe; I know of at least one case in a 12 year old cat.
Sprinkles was diagnosed at 3 and a half months. She didn’t have a particularly stressful event before developing symptoms. She’s not a purebred. I don’t know anything about her genetic history, so I can’t cross that off the list.
Mickey, my second FIP kitten, was diagnosed at 4 months. I know slightly more about his health records but it’s still scant. He arrived with an unusual skin ailment: sarcoptic mange. Hypothetically, this could indicate an already delicate immune system that left him vulnerable to this sort of FCoV mutation.
FIP is deadly and remains, to this day, the most horrifying disease I’ve ever personally encountered. Thankfully, FIP itself is NOT contagious. FCoV is highly contagious but, as previously mentioned, it’s fairly common in the cat population. There was a study done to see if separating kittens from their mother at 7 weeks (approximately the period when a mother’s antibodies begin to wear off and the kittens have to begin producing their own) would prevent cats from catching FCoV from her. This was effective but the social drawbacks are too heavy a cost for it to be considered regularly.
There is a vaccine for FCoV but it’s largely ineffective and most vets don’t recommend it.
FIP comes in two primary forms: wet (effusive) and dry (non-effusive). Usually, FCoV exists only in the gastrointestinal system. It’s really the only place it can replicate itself with ease. Once the virus mutates, it can’t replicate itself as well, but it CAN infect macrophages. Macrophages are highly mobile white blood cells. They go pretty much everywhere, and ones infected with FIPV (Feline Infectious Peritonitis Virus) will carry the virus along for the ride.
The early symptoms are vague. These cats are lethargic, listless, have low or no appetite, weight loss, and a fluctuating fever. The first symptom I caught in Sprinkles was complete avoidance: she was actively avoiding other kittens and other kittens were avoiding her. Mickey’s only symptoms were lethargy and diarrhea. I only got suspicious about possible FIP because the other kittens in his playgroup didn’t have any diarrhea at all.
And this is where we see a split in the forms of FIP.
Effusive FIP is characterized by the accumulation of fluid within the abdomen and is more common. It happens very quickly. Cats with effusive FIP develop breathing problems rapidly. The fluid drawn from the abdomen is usually straw-yellow. Effusive FIP is said to be more common, although only one of the 5 cases I’ve seen in the last few years was wet FIP.
Thankfully, effusive FIP has a few distinct traits that makes it easier to diagnose. It’s important to remember that FIP itself is generally a diagnosis by exclusion.
Measuring the protein in the effusion is a good first step. If it’s less than 35g/l, FIP is generally ruled out.
The albumin to globulin ratio is considered next, via a blood test. If it’s less than 0.4, FIP should be considered.
Finally, examining the cells in the effusion is valuable. If they’re primarily lymphocytes, FIP is excluded.
Non-effusive FIP is more difficult to spot, because the symptoms are so varied. Granulomas (inflammatory cells) form in various organs, which produces an extreme variety of symptoms. The most commonly affected symptoms are the ocular and neurological symptoms.
Ocular FIP happens when the virus crosses the blood-ocular barrier and is characterized by slightly opaque white films on one or both eyes; these don’t cover the entire eye. They’re often just a small section. This was the first distinct symptom I saw in Sprinkles. It’s considered a distinct enough sign that her ophthalmologist was able to tell me that she was 99% certain it was FIP.
Neurological FIP is my own personal hell. The virus crossed the blood-brain barrier and infects the brain. The first symptom is usually a limp or a slight tremble in the head. The paralysis often begins in the hind limbs and it travels upwards. The cat eventually loses all mobility. If the cat is lucky, they’ll begin to have seizures instead and die soon afterwards. Like I said, it remains the single-most awful thing I’ve ever seen.
Non-effusive FIP is harder to diagnose than effusive FIP, especially if the cat fails to develop ocular or neurological symptoms. In these cases, the only symptoms the cat has are fevers, diarrhea, and other non-specific issues.
Once again, the best bet is to consider the albumin to globulin ratio. The same rule of ‘if it’s under 0.4, FIP should be considered’ holds true.
Unfortunately, checking for antibodies is fairly useless. A positive FCoV test just means the cat has been exposed to FCoV.
FIP is deadly. While there are some isolated cases of cats seemingly recovering from it, I think it’s more likely that those were simply misdiagnosed cases. As I’ve said before, FIP is a diagnosis by exclusion, so a misdiagnosis can happen fairly easily. A cat with wet FIP is gone in days. A cat who’s unlucky enough to develop neurological FIP may linger for weeks until they die of starvation, oxygen deprivation as the lungs themselves are paralyzed, or dehydration. Ocular FIP generally spreads into the brain, causing seizures.
Sprinkles is very, VERY lucky. I had been following the study very closely and I had an acquaintance who recently started treating her foster cat for FIP. I was able to get into contact with some folks and obtain experimental treatment for my kittens.
GS-441525
In February 2019, there was a very promising study on a specific drug called GS-441524. Most of the cats involved with the study made a full recovery. The company (Gilead-Sciences) behind the drug wasn’t interested in getting it FDA approved for cats out of concern that it would affect its approval for human use. See, if it’s used officially for cats, Gilead-Sciences would be obligated to report any negative side-effects and that could impact getting it approved for human use down the line. “One of the rules in drug development is ‘never perform a test you don’t have to, if the results could be problematic,” isn’t an uncommon saying. It’s one of the reasons why I fell out of research and development myself.
I had some pull and was able to get experimental access to this drug for Sprinkles and, later, Mickey.
Both kittens went through three months of daily injections and a further 3 months of observations before they were deemed FIP-free. After seeing 3 other cats die from it, it’s been a blessing to see them recover. They’re both especially lucky that they finished their treatment cycle JUST before COVID-19 hit American shores since I couldn’t, in good conscience, continue using a very promising antiviral in cats when it would likely be needed by humans.
It’s definitely not a perfect system. Three months of daily injections (or pills) is not ideal for the average owner for several reasons. In addition to the difficulty of injecting a cat with an EXTREMELY painful drug daily, it also requires a lot of math; the dosage has to be adjusted daily to take weight gain into consideration. Even the concentration has to be adjusted at times. I haven’t used the pills at all, but I know a lot of people have had problems with cats biting through the pills. In addition, the pills seem less effective against neurological or ocular FIP.
Gilead-Sciences has refined GS-441524 into GS-5734 (named Remdesivir), which is supposed to be more efficient. Hypothetically, the addition of the phosphate groups should make it easier for it to get across barriers and be absorbed more easily. Hopefully this will result in a shorter treatment time, although I suspect it will be more expensive than GS-441524. This is already a substantial cost attached to GS-441524, with the treatment of a single cat or kitten over 1,000 USD.
As of writing (April 20, 2020), neither Remdesivir nor GS-441524 are available to the average public legally. Remdesivir has been approved for use in humans with COVID-19 in emergency cases.
261 notes
·
View notes
Text
Baby has a fever 😬 yesterday he had a minor rash on sole of his foot, it doesn't look like any pictures of covid rash I've seen and does look like hand foot mouth, but who could he possibly have gotten that from? He hasn't been around any kids besides M, and she is fine. He hasn't been around any people besides our family. The first few days of us being here I went to grocery stores (with the kids) but when I realized that mask compliance is shit in Oklahoma I stopped that and opted for delivery. A week ago we went to one empty playground, and Monday we went to the zoo (right when they opened, not crowded, only outdoor stuff). A goes to work, and while the job site is awful on masks (as in the person responsible for safety doesn't even wear one), the giant, totally and completely separate area he is working in is only him and 3 people from his company, who are all very serious about masks. I sanitize constantly. But we're in maskless Oklahoma with a 20% positivity rate, and covid is everywhere. As soon as I realized he had a fever today I immediately tried to get us tested. The health department website makes you answer a ton of irrelevant questions, only to say no appontments available. I tried every urgent care and was able to get an appointment for one person to be tested tomorrow and another person on Sunday. This is some random out of network questionable urgent care place, who even knows if they're using FDA-approved tests, but I was desperate. After 2 hours on hold with the university (which provides free, legit testing, a block from our house), I was finally able to get little guy and I drive through tests this afternoon. Results should be available in 24-48 hours. I am really worried about him, sick babies are so sad and scary. Luckily there is instacart here, so I'm getting Motrin delivered, because Tylenol isn't cutting it (fever is still 102.5, dropped to 100 after Tylenol, then back up an hour later). I would never really do it, I know this would be insane horrific Trump-level public health behavior, but the thought definitely did cross my mind to buy a plane ticket and bring my sick maskless baby home to New York. I would never really do that. But I definitely miss our lovely pediatrician who knows us and adores him and tests kids no prob in her office, NY where it's easy to get a rapid test, where hospitals aren't full and people believe covid is real and wear masks. For what it's worth, A says I'm working myself up (I was convinced I had covid shortness of breath, because I was having an anxiety attack during the two hours on hold to make an appointment) and baby will be fine tomorrow.
34 notes
·
View notes
Text
Diary of an Anxious Person Getting Her First Covid Vaccine

- Awoke at 5 a.m, two hours before the alarm, and couldn’t fall back to sleep. Why? WE KNOW WHY.
- Put on extra deodorant because, well, stress.
- Drank LOTS of water because Mom said it might help. She’s been telling me this since I exited her womb and after 51 years I finally decided it might be a good idea.
- Arrived at the medical facility a half hour early (because one can never be too early for things that terrify us). Sat in the car for 15 minutes, stomach churning, heart palpitating and also wondering what they serve at the cute little diner on the corner. Considered canceling my vaccine appointment but want to be able to attend future 80s hair band concerts without risk of covid infection so in the name of Tommy, Gina and all others who are Livin’ on a Prayer I bravely stepped out of my car.
- Entered the doctor’s office and found some relief in the fact that hardly anyone else was there (fewer people to witness the fainting spell that was soon likely to occur).
- Listened to the balding guy in front of me joke to the receptionist that she has plenty of room up on his forehead to take his temperature. Decided that if Balding Guy and I are going to die next to each other in Vaccination Land, at least he will make me laugh as we go out.
- Decided not to read the covid vaccine pamphlet that was handed to me because the mere mention of side effects would most certainly guarantee I will experience every single one of them. This is The Law of Anxiety. Plus, I’ve had them memorized for months.
- Stood on the first “X” of three taped on the floor outside of the patient rooms, waiting for the nurse to take me back for my shot. Silently congratulated myself for putting on extra deodorant.
- Looked deep into the nurse’s eyes to see if she looked competent to administer the lethal injection. She barely passed the test...but I liked her hair.
- Found out the nurse hadn’t personally been vaccinated yet. She was supposed to get the Johnson & Johnson vaccine because she hates shots and that only requires one shot not two. Now that Johnson & Johnson’s is temporarily off the market, it’s a future two-fer vax for this nurse. Felt immediate bond with her because we share a fear of shots - and, judging by her ‘do, a love of big hair. Began chatting with her like I was her new bestie. Made awkward jokes. Wondered if Balding Guy was making his nurse laugh. Probably a lot more. I decided to shut up.
- Felt the long-awaited needle penetrate my left arm muscle. Waited for Death to arrive.
- Wondered why I was still conscious. Rose from my seat and followed the nurse into the hallway where fellow vaccine-ers were sitting in socially distanced chairs for 15 minutes of “observation.” My hypervigilant brain began scanning my body for any unusual sensations or signs of my early demise. Nothing - except a desperate need to pee.
- Got text from childhood friend asking how I was doing. I explained that I was trying to remain upright. Friend felt this was an impressive goal. We discussed the vaccine and anxiety to keep my mind off the vaccine and anxiety as I waited to pass the crucial 15-minute mark. If I had no serious reaction to the vaccine after 15 minutes I would be allowed to go home. Home! I tried not to imagine the vaccine seeping into my organs. I wondered what other things I have taken that are not officially “FDA-approved.” Vodka?
- The nurse called several others under observation one-by-one to her station to ask them questions before allowing them to leave: What is their birthdate? Are they finding it hard to breathe? Any dizziness? And just like that, I forgot if and when I was born, gasped for breath and grabbed the edge of my chair to stop the room from spinning. Wait - no, I was fine. Good one, anxiety. Good one.
- Balding Guy got called by the nurse. He asked “how much vaccinated” he is now. The nurse guessed 60 percent. My brain channelled the Hunger Games: “May the odds be forever in your favor.”
- Finally, I was called. I heard myself answer the nurse’s questions correctly as my brain screamed, “Son of a gun! You are gonna make it, you crazy kid! You are really GONNA MAKE IT!!!”
- Deemed suitable to return to society, which I found quite concerning if not downright negligent, I was released from observation. I raced to the nearest bathroom, where my bladder let out a huge thank you. Once again, Anxious Brain scanned my body for any signs of vaccination distress. Maybe death comes at 30 minutes, not 15?
- Drove home, listening to the 90s on 9 channel to reassure myself that if Britney Spears and her boyfriend can make it through covid vaccinations without any drama, so can I. That’s right - work, b**ch.
- My new vaccination card is a reminder that in one month I have to do this all over again. Talk about a Debbie Downer. But hey, at least I’m halfway done with this process, which means I’m halfway back to hair band concerts. Balding Guy, see you again on May 14th - and maybe at a future hair band concert (I hope he sees the irony in that last statement).
Hair’s to a freer, healthier, safer summer!
#covid#moderna#vaccine#vaccination#anxiety#fear#medical#health anxiety#shots#hair bands#diary#nurse#balding#psychosomatic#observation#water#hydration#humor#Johnson & Johnson#brain games#doctor#living on a prayer#pandemic#hypervigilance#Britney Spears#Bon Jovi
7 notes
·
View notes
Video
youtube
What Will it Take to Create a Vaccine for COVID19?
April 29, 2020, 3:30 p.m. — One of the big challenges in navigating the COVID-19 crisis is the novelty of the virus. Because it’s so new, we don’t have any way to prevent infection — and without a vaccine, there’s no telling how long the pandemic will last. So what will it take to get a COVID-19 vaccine available to the public? And why is it taking so long?
What we know about COVID-19
Viruses operate by attaching themselves to living cells in our body and injecting their genetic material, called RNA. The RNA hijacks the machinery of the cell to replicate itself, producing copy after copy of the virus, which eventually explode out of the cell to go in search of new cells to infect. In the case of SARS-CoV-2, the virus primarily attacks the epithelial cells lining the lungs. In the lungs, the fight between your immune system and the virus can cause inflammation, cell death and excess fluid, a combo that leads to pneumonia. Sometimes, when the infection is really bad, patients end up with acute respiratory distress syndrome, or ARDS, when their lungs are so clogged up that their bodies aren’t able to absorb enough oxygen and they really struggle to breathe.
There’s also evidence that the virus, and/or the inflammation it causes, might be having effects on other organs in the body, causing long term complications including brain, liver, kidney, and heart damage and increased risk of blood clots.
Protection against a viral illness is conferred by antibodies, produced by the immune system in response to exposure to the virus. In most cases, contracting the virus gives the immune system the information it needs to produce those antibodies, which can then activate and fight the virus on the next exposure.
What it takes to develop a vaccine
Vaccines take advantage of our natural immune system response to stimulate antibody production against a disease using killed or weakened versions of the pathogen that don’t make us sick. This makes us immune to the disease without ever having to contract it.
The first step in creating a vaccine is to identify a good antigen to target. These molecules represent portions of the virus’ structure that don’t cause illness by themselves but do activate the immune system to produce the correct antibodies. These antigens are then produced in large quantities by cells in Petri dishes before being purified and, if necessary, the virus is inactivated or killed so it can be safely injected. Finally, the antigen is mixed with adjuvants, to help enhance the immune response to produce stronger immunity, alongside preservatives and stabilizers to give the vaccine a long shelf life and allow for multiple doses from the same vial.
Once a vaccine has been created, it has to be extensively tested: first in animal models, and eventually in human beings. This process goes through three phases, to be sure that the vaccine is safe and effective, and to determine what dose should be administered to patients. It takes a lot of testing to be sure that the vaccine is safe in children, adults, pregnant people, and the elderly. And not every vaccine is recommended for every person. Different governments have to balance cost, risk, efficacy, and public health concerns before deciding whether or not they should add a new vaccine to the standard vaccination list.
Usually, it takes 10 to 15 years to develop a vaccine and get it approved by the FDA for use in patients. The exception are seasonal vaccines, like the flu vaccine. Because the flu virus is tracked year-round by scientists, and because the manufacturing process is kept the same year-to-year, researchers are able to use the same foundation for each year’s vaccine and simply replace the antigen with the new virus.
In the case of SARS-CoV-2, because of the high rates of infection and novelty of the virus, it seems critical that we get a vaccine ready quickly, but it’s not as easy just swapping in a new antigen. Thanks to a lot of technological advancement in recent years, and the large number of industrial and academic researchers working on this problem, we’re moving faster than ever toward a vaccine solution for COVID-19.
What’s already happening on the vaccine front?
The SARS-CoV-2 genetic sequence was published on January 11, 2020, allowing for a global flurry of vaccine development, and the first vaccine entered human clinical trials on March 16. By April 8, 115 vaccines candidates were being tested by academic research institutions and industry companies, with 78 of those candidates being close to formal testing. Several of those have already advanced into clinical testing, with several others hoping to follow within the next several months.
The rapid speed of vaccine development is thanks to a wide diversity of technology platforms, testing a variety of potential sources for stimulating the immune response, including peptides, viral vectors, recombinant proteins, and nucleic acids (DNA and RNA). Researchers are also working to develop adjuvants that will produce the most effective vaccines. Most of these vaccines are being tested in the United States and in China, with some development in Europe, Australia, and other areas in Asia.
What we still need to know and do
We still need to figure out some key details about the SARS-CoV-2 virus. One big question that we haven’t answered yet is whether or not exposure to the virus, or to viral antigens, is enough to generate immunity to the virus. The evidence so far suggests that people who have contracted COVID-19 are protected against reinfection, at least temporarily.
Some cases have indicated that patients who experience a more severe infection of the virus may have more antibodies against it, which may provide better protection against future illness. But it’s still unclear if contracting the virus provides any lasting immunity against it, or how long that immunity might last. At this point, virologists believe that immunity is limited; after 1 to 2 years, patients may be susceptible to a new infection, and require a vaccine to protect against it.
Another question is whether or not COVID-19 is at risk of mutating to become more deadly. At this stage, we just don’t know enough about the virus and its behavior to be certain one way or the other, though some experts doubt that there is a high risk of this happening.
Until some of these vaccines have gone through further clinical testing, we won’t know which ones are actually viable candidates for the public. Part of the challenge is the fact that most vaccines require years of testing in the community, where trial participants receive the vaccine or receive a placebo and then go about their daily lives to see if they end up contracting the virus. Because of the drastic impacts of COVID-19 on our lives and economy, some scientists are suggesting that we consider clinical trials where healthy volunteers are given the vaccine and deliberately exposed to SARS-CoV-2 to see how effective the vaccine might be. This could shave months off the development timeline, but is highly controversial due to the obvious risks for trial participants.
So when will we have a vaccine?
With all of these projects underway, scientists think a vaccine may be approved for emergency use by the FDA as soon as 2021. “Emergency use” means that a vaccine has been tested in humans and found to be effective at preventing infection, but hasn’t been subjected to the full, rigorous scrutiny of a traditional vaccine. It will take more time for all of this extensive testing to be completed — likely several more years.
Despite recent technological advances, this means it will still be at least a year before we have a viable vaccine. With that in mind, the CDC and other public health officials continue to recommend that we follow physical distancing, hand washing and mask-wearing guidelines. It’s important that we continue to work together to protect the health of our friends, family, and community by following stay-at-home recommendations as doctors and scientists work not only on developing a vaccine, but also at better understanding how the virus works and figuring out the most effective ways to treat it.
For more information on what we’re doing at UC San Diego Health, please visit health.ucsd.edu/COVID
— Alison Caldwell, PhD, Bigelow Science Communication Fellow
#covid-19#covid 19#novel coronavirus#coronavirus#sars-cov-2#vaccine#immunology#infectious disease#public health#clinical trials#vaccine protocol#vaccine production#stay at home#flatten the curve#pandemic#hand washing#hand hygiene#shelter in place#self quarantine#quarantine#social distancing#science#medicine#academic medicine#ucsd#uc san diego
59 notes
·
View notes
Text
How does the coronavirus test work? 5 questions answered
by Maureen Ferran

The U.S. has been scrambling to get testing for the coronavirus up to speed. AP Photo/Francois Mori
The U.S. government is fighting to contain and slow down the spread of the coronavirus. Testing is central to these efforts. Molecular biologist and viral researcher Maureen Ferran answers some basic questions about how these diagnostic tests work – and if there are enough to go around.
Who gets tested for the virus?
Currently there are two main reasons someone would be tested for the coronavirus: having symptoms or exposure to an infected person.
The main symptoms of COVID-19, the disease caused by the coronavirus SARS-CoV-2, are fever, dry cough and shortness of breath. These look a lot like the flu and the common cold, so it takes a physician to determine if testing for the virus is necessary.
Initially, the Centers for Disease Control and Prevention recommended testing only people with symptoms and who had potentially been exposed to the virus. But to the surprise of public health officials, several of the first people in the U.S. who tested positive for the virus had no obvious exposure. This development suggested that the virus was being transmitted locally, meaning it was spreading from person to person easily and/or that people may have been transmitting the virus without experiencing serious symptoms.
In response, on March 4 the CDC changed its recommendations to allow anyone with COVID-19-like symptoms to be tested as long as a doctor approved the request. Since the number of available tests is limited, the CDC is encouraging physicians to minimize unnecessary testing and consider a patient’s exposure risks before ordering tests.
As of writing this, there are no specific treatments available for COVID-19, but that does not mean testing is pointless. Perhaps most importantly, testing is done so that infected patients can be quarantined and the spread of the virus slowed. Another benefit of testing is that it lets public health workers build a more accurate picture of the number of cases and how the virus is spreading in the population.

Taking a sample is quick, easy and can be done anywhere. Finding out if a person is infected is more complicated. Rodolfo Parulan Jr./Moment via Getty Images
What it is like to get tested?
For a patient, the process of being tested for the virus is easy and can potentially be done almost anywhere. It typically involves taking a swab from deep in a patient’s nasal cavity to collect cells from the back of the nose. The sample is then sent to a lab, where it will be tested to determine if the patient’s cells are infected with the virus. The same process is used to collect a sample from a patient who is tested for flu.
How does the test work?
While collecting a sample is easy, actually determining whether a person is infected with the coronavirus is much more complicated. The current method looks for the virus’s genetic material (RNA) in a patient’s cells.
In order to detect the presence of RNA in the patient’s sample, labs perform a test called reverse-transcription polymerase chain reaction. This method first converts any viral RNA to DNA. Then the DNA is replicated millions of times until there are enough copies to detect using a specialized piece of equipment called a quantitative PCR instrument.
If genetic material from the virus is found in the sample, then the patient is infected with the virus.
It takes 24-72 hours to get the results of a test. During the early ramp-up of testing, there were some concerns about the test’s accuracy after one study found 3% of tests in China came back negative when the samples were actually positive. But this type of genetic test is generally very accurate – more so even than rapid flu tests – and the benefits of testing outweigh the risk of an error.

As the virus spread, the testing capacity needed to grow, and fast. AP Photo/Andrea Casalis
Does the US have enough tests?
The availability of tests has been a big issue. Prior to Feb. 29, the CDC was the only place approved by the FDA to develop, produce and process tests. However, as the number of suspected cases climbed and doctors approved more people for testing, demand to be tested soared.
The test for the coronavirus requires a kit, specialized equipment and specially trained personnel. Faulty and slow development of test kits and the initial requirement that all tests be processed at the CDC contributed to the slow rollout across the U.S.
As pressure on the federal government to make tests available increased, the FDA announced a new policy on Feb. 29 that made it easier for commercial and academic laboratories to develop their own tests and allowed other certified labs to test patient samples.
Integrated DNA Technologies, a CDC contractor, shipped 700,000 tests to commercial, academic and health care laboratories on March 6. Quest Diagnostics and LabCorp, two large commercial test manufacturers, started making their own test kits, which became available on March 9. Many companies, hospitals and other institutions are now racing to develop more tests to diagnose COVID-19.
On March 10, Alex Azar, secretary of Health and Human Services, announced that 2.1 million testing kits are now available and more than 1 million have shipped to certified labs for testing. Millions more are expected to ship out this week.
Does everyone really need to be tested?
Realistically, it isn’t feasible to test everyone who is sick in the U.S. Therefore, most health officials believe it is important to prioritize the testing of people who need it the most: those at high risk such as health care workers who have been in contact with COVID-19 patients; symptomatic people in areas with high infection rates; and people 65 years of age and older with chronic health issues, such as heart disease, lung disease or diabetes. As more tests become available, it will be possible to test more people.
There’s also a need to develop faster tests that do not require special equipment and personnel. Testing allows experts to better understand how the outbreak is progressing and try to predict the impact the virus will have on society.
As with all outbreaks, this pandemic will end. In the meantime, however, people need to wash their hands and try to minimize their risk of exposure. There is much to be learned about this novel coronavirus. Only time will tell if it disappears from the human population, as SARS did in 2004, or becomes a seasonal disease like flu.

About The Author:
Maureen Ferran is an Associate Professor of Biology at the Rochester Institute of Technology
This article is republished from our content partners over at The Conversation under a Creative Commons license.
16 notes
·
View notes
Text
Latest Management Strategies for Hospitalized COVID-19 Patients
The disease spectrum of COVID-19 is very wide. On the one hand are the asymptomatic or mildly symptomatic patients and on the other hand are patients who are fighting for their lives in the intensive care units. A significant number of hospitalized patients undergo severe inflammatory reactions triggered by SARS-CoV-2. Besides supportive therapy, more aggressive treatment strategies are being increasingly used with variable degrees of success.
Blood Purifying Devices
Extracorporeal blood purification (EBP) is a treatment in which blood from a patient is passed through a device (e.g. a membrane or a sorbent) in which a solute (waste products, toxins) and possibly water are also removed. When fluid is also removed, replacement fluid is added. EBP is used primarily in patients with renal failure (Renal Replacement Therapy-RRT). More than twenty years ago, it was seen that RRT could also remove inflammatory mediators from the plasma of septic patients. A survival benefit with hemofiltration was also noted later. Hence, started the use of blood purification as a treatment for human septic shock.
The role of cytokine storm and hyper inflammation, on the severity of COVID-19 patients has been well corroborated in different studies. In an article published in Intensive Care, Ruan et al documented high concentrations of inflammatory biomarkers like C- reactive protein, ferritin and IL-6 in COVID-19 patients who died. They concluded that COVID-19 mortality may be due to virus activated “Cytokine Storm Syndrome” or fulminant myocarditis.
Blood purification devices have now been given permission for use in severe cases of COVID-19 in a number of countries. One brand of blood purifying devices, CytoSorb, was used to treat more than seventy critically ill COVID-19 patients and specifically added to Coronavirus treatment guidelines in Italy and Panama, March 25, 2020. The use of CytoSorb has now begun in seriously ill COVID-19 patients who have cytokine storm and life threatening complications such as Acute Respiratory Distress Syndrome (ARDS), in Italy, China, Germany and France. Preliminary data of the seventy patients tested, shows that there is a marked reduction in Cytokine storm and inflammation, improved lung functions, weaning from mechanical ventilation and a reversal of shock.
CytoSorb is now specifically recommended in the Italy Brescia Renal COVID Task Force Guidelines for treating patients with severe COVID-19 infection and stage 3 renal failure on continuous RRT, published in Italian Society of Nephrology website. Blood purification in COVID-19 infections is also recommended to treat Cytokine Storm by the National Health Commission in China. This technology has been used in thousands of Extra Corporeal Membrane Oxygenation (ECMO) treatments to date, in non COVID-19 patients around the world.
On April 10, 2020, US FDA gave emergency use authorization (EUA) for a pair of Blood Purification Systems to treat adult COVID-19 patients admitted in ICU. The EUA applies to Trumo BCT Inc’s Spectra Optia Apheresis and Marker Therapeutics AG’s Depuro D2000 Adsorption Cartridge. On April 13, 2020 it gave EUA for the CytoSorbent System too.
Mesenchymal Stem Cell Transplantation (MSC)
Umbilical Cord derived Mesenchymal Stem Cells (MSCs) have been used safely and effectively for immune mediated inflammatory diseases such as Graft Versus Host Disease (GVHD) and Systemic Lupus Erythematosus (SLE). MSCs play a positive role in two ways, by immunomodulation and by their ability to differentiate. Immunomodulatory actions are related to secretion of cytokines and because of the direct interactions with immune cells. The immunomodulatory effects are further enhanced by the activation of Toll-Like Receptors (TLR) in MSCs by pathogen associated molecules such as Lipopolysaccharides (LPS) or single stranded RNA from viruses like SARS-CoV-2.
The first step in the pathogenesis of COVID-19 is the entry of SARS-CoV-2 into human cells by attaching to Angiotensin Converting Enzyme-2 (ACE-2) receptors by its spike proteins. A research team from Germany revealed that cellular serine protease TMPRSS2, for SARS-CoV-2 Spike Protein priming, is also important for host cell entry and spread.
ACE2 receptors are present widely on tissues in the body, like type 2 Pneumocytes of lungs, kidney, intestines, capillary endothelium etc. However, the immune cells such as T and B lymphocytes and macrophages, in the bone marrow, lymph nodes, thymus, and the spleen, are all negative for ACE-2 receptors. MSCs are also ACE-2 receptor and TMPRSS2 negative. This makes them immune to SARS-CoV-2. This is the basis of using them in severe COVID-19 infections.
The viral infection causes a total failure of function of lymphocytes and almost the whole immune system. Several studies have reported lymphopenia and high levels of C-reactive protein in COVID-19 patients with severe infections. MSCs play a role by reversing the lymphocyte subsets, mainly through dendritic cells. The interactions of MSCs with the dendritic cells leads to a shift of the immune system from T helper 1 to T helper 2 type of responses.
After entering the human body through the intravenous route, part of the MSCs accumulate in the lung. There, they probably improve the microenvironment, protect alveolar epithelial cells, prevents pulmonary fibrosis and improve lung functions. Due to their immunosuppressive capacity, MSCs significantly decrease the serum levels of pro inflammatory cytokines and chemokines. This leads to decreased attraction of mononuclear/macrophages to the fragile lung, at the same time recruiting more regulatory dendritic cells to the sites of inflammation. They also increase IL-10 and Vascular Endothelial Growth Factor (VEGF), which promotes lung repair.
US FDA has authorized Umbilical cord derived Mesenchymal Stem Cell (MSC) transplant, to prevent life threatening lung inflammation that accompanies severe cases of COVID-19. They have provided authorization for a twenty four patient clinical trial for such patients.
An article related to the use of MSCs in COVID-19 pneumonia, was published by Leng Zikuan, Zhu Ronjia, Hou Wei, et al in the Journal of Aging and Disease, February28, 2020. They studied the effects of MSC transplant on seven COVID-19 patients with pneumonia. A favorable result was reported in all of them.
Use of Convalescent Sera in COVID-19 Patients
Immunity is of two types- active and passive. Active immunity is when the human body mounts an immune reaction in response to an invading microorganism. Passive immunity is when the body is not actively involved in producing immunity. This involves introducing preformed antibodies into the human body through various routes. A classic example of this is the newborn receiving maternal antibodies that protect the newborn till the age of around six months.
Convalescent Sera has been used as early as the twentieth century, to stem the outbreak of viral diseases such as poliomyelitis, measles, mumps and influenza. In the 2009-2010 H1N1 influenza virus pandemic, convalescent sera was used to treat patients with severe H1N1 disease, requiring intensive care. Convalescent serum was also used in the 2013 West African Ebola Virus epidemic. In previous epidemics of Coronaviruses, SARS 1 in 2003 and MERS in 2012, Convalescent Sera was used for patients who were hospitalized.
Recently on April 13, 2020, US FDA issued guidelines to health care providers and investigators on the administration and study of convalescent plasma, collected from individuals who have recovered from COVID-19. COVID-19 convalescent plasma has not yet been approved for use by FDA. It is regulated as an investigational product. Eligibility criteria for the potential patients:
Laboratory confirmed COVID-19.
Severe or immediately life threatening COVID-19, for example:
Informed consent provided by the patient or a health care proxy.
Severe disease is defined as one of the following:
Life threatening disease is defined as one or more of the following:
Shortness of breath (dyspnea)
Respiratory frequency ≥ 30
Blood oxygen saturation ≤ 93%
Ratio of partial pressure of arterial oxygen to fraction of inspired oxygen < 300.
Lung infiltrates > 50% within 24-48 hours.
Respiratory failure.
Septic shock
Multiple organ dysfunction or failure.
So, the research continues, as the pandemic spreads. Scientists, epidemiologists and researchers are working at a fast and furious pace along with the governments of their countries, to expand their knowledge of the disease, its diagnosis and management.
HEALTH DISCLAIMER
This blog provides general information and discussions about health and related subjects. The information and other content provided in this blog, or in any linked materials, are not intended and should not be construed as medical advice, nor is the information a substitute for professional medical expertise or treatment.
The content is for information purpose only and is not a medical advice. Qualified doctors have gathered information from reputable sources; however Credence Medicure Corporation is not responsible for errors or omissions in reporting or explanations. No individual should use the information, resources and tools contained herein to self diagnose or self treat any medical condition.
If you or any other person has a medical concern, you should consult with your health care provider or seek other professional medical treatment. Never disregard professional medical advice or delay in seeking it because of something that have read on this blog or in any linked materials. If you think you may have a medical emergency, call your doctor or emergency services immediately.
The opinions and views expressed on this blog and website have no relation to those of any academic, hospital, health practice or other institution.
Credence Medicure Corporation gives no assurance or warranty regarding the accuracy, timeliness or applicability of the content.
1 note
·
View note
Text
Treatment options available for COVID-19
As the Covid-19 pandemic continues to spread around the world. The viral infection infected millions of people around the world. Doctors, scientists, and governments are collaborating around the world to look for methods to tackle the pandemic.
The Covid-19 belongs to the Coronavirus family that causes illness such as the common cold, SARS, and MERS. The newest strain of Coronavirus originated in Wuhan, China. In March 2020, the World Health Organization (WHO) declared Covid-19 a pandemic.
Covid-19 s extremely infectious and spreads by the respiratory droplets when an infected person coughs or sneezes or talks. The virus can survive on surfaces and can spread if a person touches his mouth, nose, or eyes after touching a contaminated surface.
Symptoms of Covid-19 are more severe in older adults or those with underlying health conditions. Common symptoms of Covid-19 are:-
Fever
Cough
Breathing difficulty
Fatigue
Other symptoms of Covid-19 may include:-
Body ache
Loss of smell and taste
Headache
Diarrhea
Vomiting
If you have had contact with a Covid-19 patient or have had a recent travel history, book a doctor in Pune immediately.
Most people with Covid-19 will recover at home. No specific treatment for the virus exists as of now and the best doctor in Pune will try to prescribe medication to alleviate your symptoms. Rest, keeping yourself hydrated, and a supplementary source of oxygen may be required sometimes.
Currently, there are no antiviral medications to treat Covid-19. Drugs developed to treat other viral infections are being tested to see if they work against Covid-19. Some options being explored.
Remdesivir was first developed to treat Ebola. Researchers found that using Remdesivir was effective in fighting the Covid-19. Clinical trial in China has been successful. FDA has approved clinical studies to be carried out in the USA. ICMR may use the drug if local drug companies get approval to manufacture it.
Chloroquine is traditionally used to fight malaria and autoimmune diseases like Lupus. Doctors in several countries including India have used Chloroquine effective against severe symptoms of Covid-19. The ICMR (Indian Council of Medical Research) has been used to treat asymptomatic medical staff treating COVID-19 or asymptomatic households with confirmed cases. Doctors caution patients about using the medication without doctor’s supervision due to the risk of heart rhythm problems.
Lopinavir and ritonavir are used to treat HIV. The medicine is sold under the brand name Kaletra. The WHO indicates that Kaletra has benefits when used in combination with other drugs that have been found to be effective in treating COVID-19.
Flavipir is a Japanese antiviral drug used to treat influenza in Japan. India is starting clinical trials to treat COVID-19. Flavipir is still not part of WHO solidarity trials.
Plasma therapy has shown tremendous promise in treating Covid-19 patients with severe symptoms. When a person has recovered from Covid-19 their blood contains antibodies used to fight the viral infection. We find these antibodies in plasma (a component of the blood). Patients who have recovered can donate their plasma via transfusion. The donor antibodies can help patients fight the disease faster. Plasma therapy has been approved by the ICMR and is being used in several states.
Research is underway to produce a vaccine to treat Covid-19. The Serum Institute in Pune is collaborating with Oxford to produce the vaccine.
1 note
·
View note
Quote
08:34 (IST)
Coronavirus Outbreak in West Bengal Latest Update
Bengal govt forms teams for surveillance support, monitoring of treatment at COVID-19 hospitals
The West Bengal Health Department on Saturday formed teams to support surveillance and monitoring of treatment at five hospitals treating COVID-19 patients in the city. The team members will pay regular visits to these hospitals and send reports to the department, the state government said in an order.
The department has also set up a dedicated helpline for issues regarding the non-availability of PPEs and other supplies. The feedback and suggestions will be duly recorded and acted upon by the state government for appropriate remedial measures, the order said.
08:29 (IST)
Coronavirus Outbreak in India Latest Update
IMAGES: Indians stranded in Uzbekistan prepare to leave for New Delhi
Indians stranded in Uzbekistan due to COVID-19 outbreak, leaving for New Delhi under Vande Bharat Mission special mission prepare for take off. Thermal checks being performed by the Indian Embassy officials in Tashkent, Santosh Jha, Ambassador of India to Uzbekistan told ANI.
Indians stranded in Uzbekistan due to #COVID19 leaving for New Delhi under #VandeBharatMission. Thermal checks being performed by the Indian Embassy officials in Tashkent: Santosh Jha, Ambassador of India to Uzbekistan pic.twitter.com/3RnrWA0KD0
— ANI (@ANI) May 10, 2020
08:21 (IST)
Coronavirus Outbreak in US Latest Update
US approves new coronavirus antigen test with fast results
US regulators have approved a new type of coronavirus test that administration officials have promoted as a key to opening up the country, reports The Associated Press.
The Food and Drug Administration on Saturday announced emergency authorization for antigen tests developed by Quidel Corporation of San Diego. The test can rapidly detect fragments of virus proteins in samples collected from swabs swiped inside the nasal cavity, the FDA said in a statement.
The antigen test is the third type of test to be authorized by the FDA.
08:15 (IST)
Coronavirus Outbreak in Maharashtra Latest Update
Mumbai Police pays tribute to cop who died of COVID-19 on Twitter
The Mumbai Police on Sunday paid tribute to assistant sub-inspector (ASI) attached to Vinoba Bhave Nagar police station who died of the coronavirus disease COVID-19 on Friday.
The samples of an assistant sub-inspector attached to Vinoba Bhave Nagar police station in Mumbai who died on Friday have tested positive for novel
coronavirus, PTI quotes officials as saying.
He died in the early hours of Friday after being admitted in a civic hospital on Wednesday with COVID-19-likesymptoms, an official said.
Mumbai Police regrets to inform about the unfortunate demise of ASI Sunil Dattatray Kalgutkar from Vinoba Bhave Nagar Police Station. ASI Kalgutkar had been battling Coronavirus.
We pray for his soul to rest in peace. Our thoughts and prayers are with the Kalgutkar family.
— Mumbai Police (@MumbaiPolice) May 9, 2020
08:10 (IST)
Coronavirus Outbreak in Assam Latest Update
Not testing samples of 16-yr-old girl who died of COVID-19 was 'a mistake', says Himanta Biswa Sarma
Doctors at the government-run ESIC Hospital here committed a "mistake" by not testing samples of a 16-year-old girl, who later died of Covid-19, Assam Health Minister Himanta Biswa Sarma said on Saturday.
The girl breathed her last on Thursday at the B Barooah Cancer Institute. Her samples, which were taken after her death, tested positive for coronavirus infection/
"The girl first went to ESIC Hospital with all symptoms like fever and pain in legs. It was a mistake by doctors that her samples were not sent for testing... It is a matter of concern," Sarma told reporters.
08:05 (IST)
Coronavirus Outbreak in Delhi Latest Update
Delhi school teacher involved in distributing ration tests positive for COVID-19
A teacher of a civic body-run school, who was involved in distributing ration during the lockdown, has tested positive for the novel coronavirus, officials said on Saturday. The teacher was posted at a primary school in Wazirabad under the North Delhi Municipal Corporation.
The teacher had last come to school on April 28 and started showing COVID-19 symptoms from 2 May. His test report came on Friday, an official of North Delhi Municipal Corporation said.
"We traced his six primary contacts and they have been sent into quarantine. Since they are completely asymptomatic, no test has been done yet," he said, adding the school building has been sanitised.
07:57 (IST)
Coronavirus Outbreak in Pakistan Latest Update
Pakistan eases nationwide lockdown even as COVID-19 cases rise
Pakistan on Saturday began easing the month-long lockdown despite a steady rise in the number of coronavirus cases which has now crossed the 28,000-mark with 618 deaths.
Doctors have warned against easing restrictions. The Representative of Pakistan Medical Association (PMA) has demanded that the government observe World Health Organisation protocols and implement strict lockdown.
"We think the number will definitely spike. According to our information, there are five hospitals in Karachi that have a total of 63 beds reserved for coronavirus patients. If this is the condition in a city like Karachi, then you can imagine what it is like in other cities of Pakistan," said Dr Ikram Tunio of the PMA in a press conference in Karachi.
07:47 (IST)
Coronavirus Outbreak in India Latest Update
First batch of 326 Indians stranded in UK arrives in Mumbai
The first batch of 326 Indian nationals stranded in the UK due to the coronavirus-linked global travel restrictions arrived here from London early on Sunday morning.
The special evacuation flight AI 130, a Boeing 777 plane which departed from London on Saturday, landed at Mumbai's Chhatrapati Shivaji Maharaj International Airport (CSMIA) at around 1.30 AM with 326 Indians, according to a source.
The airport authorities, in a statement on Saturday, said that the arriving passengers with symptoms will be moved to isolation centres.
Asymptomatic passengers residing in Mumbai will be moved to quarantine facilities like hotels, while those from outside of the city will be transported by the state to their respective district headquarters, it said
Maharashtra: A special flight carrying Indian nationals from London, arrived at Chhatrapati Shivaji International Airport in Mumbai tonight. #VandeBharatMission pic.twitter.com/2VhXl72Y4r
— ANI (@ANI) May 9, 2020
07:41 (IST)
Coronavirus Outbreak in India Latest Update
CRPF reports 62 new cases on Saturday
As many as 62 more personnel of Central Reserve Police Force (CRPF) have been infected with coronavirus on Saturday.
"With 62 new COVID-19 cases, the total number of coronavirus cases reported from CRPF is 234, of which 231 are active cases," according to an official statement issued by the CRPF.
Meanwhile, 35 new cases of COVID-19 have been reported in Border Security Force (BSF) on Saturday, taking the total count of cases in the force over 250.
07:35 (IST)
Coronavirus Outbreak in India Latest Update
Air India flight with 163 Indians from Kuwait lands at Hyderabad airport
An Air India flight with 163 Indians landed at the Hyderabad international airport from Kuwait on Saturday night as part of the government's Vande Bharat Mission to bring home Indian nationals stranded abroad, airport sources said. The AI flight 988 landed at the airport shortly after 10 pm, the sources said.
To facilitate the arriving passengers and aircraft crew, the Hyderabad International Airport has kept the international arrivals and the stretch right from the aerobridge to the arrivals ramp fully sanitized and fumigated, the sources said.
07:33 (IST)
Coronavirus Outbreak in Odisha Latest Update
Odisha registers 58 new cases, taking total to 352
Odisha on Sunday reported 58 more COVID-19 cases. The total number of cases in the state is now at 352, including 281 active cases, 68 cured/recovered & 3 deaths, according to the latest date by the state health department, reports ANI
07:31 (IST)
Coronavirus Outbreak in India Latest Update
First Air India repatriation flight takes off from US with 224 Indians
Around 224 Indians stranded in the US due to the coronavirus-induced lockdown boarded the first repatriation flight from San Francisco to Mumbai and Hyderabad on Saturday.
In the first phase of the US-India segment of the 'Operation Vande Bharat- A homecoming', flights have been planned from San Francisco, Chicago, New York, and Washington DC to New Delhi, Mumbai, Hyderabad, Ahmedabad, Chennai and Bengaluru.
As many as 1,961 Indians are likely to be repatriated through seven flights from the four cities in the first phase, officials said.
07:20 (IST)
Coronavirus Outbreak in India Latest Update
Total COVID-19 cases in India now at 59,662 with nearly 2,000 deaths
The nationwide tally of confirmed COVID-19 cases reached 59,662 on Saturday and the death toll rose to 1,981 with the country registering an increase of 95 deaths and 3,320 cases in 24 hours till Saturday morning, the Union Health Ministry said even as fresh infections among central armed police forces, and the repatriated Indians raised new concerns among experts.
The number of active COVID-19 cases stood at 39,834, while 17,846 people have recovered and one patient has migrated, the Union Health Ministry said.
Coronavirus Outbreak LATEST Updates: The first batch of 326 Indian nationals stranded in the UK due to the coronavirus-linked global travel restrictions arrived here from London early on Sunday morning. The special evacuation flight AI 130, a Boeing 777 plane which departed from London on Saturday, landed at Mumbai's Chhatrapati Shivaji Maharaj International Airport (CSMIA) at around 1.30 AM with 326 Indians, according to PTI source.
The nationwide tally of confirmed COVID-19 cases reached 59,662 on Saturday and the death toll rose to 1,981 with the country registering an increase of 95 deaths and 3,320 cases in 24 hours till Saturday morning, the Union Health Ministry said even as fresh infections among central armed police forces, and the repatriated Indians raised new concerns among experts.
The number of active COVID-19 cases stood at 39,834, while 17,846 people have recovered and one patient has migrated, the Union Health Ministry said.
Saturday also saw Maharashtra crossing the 20,000 mark, as per official figures released by the state health department, with the state now accounting for one-third of the total confirmed cases in the country, while large numbers of cases continued to get detected in Gujarat, Delhi and Tamil Nadu.
Meanwhile, the Union Health Minister Harsh Vardhan said the testing capacity for COVID-19 has been ramped up to around 95,000 tests per day and a total of over 15 lakh tests have been conducted so far across hundreds of government and private labs.
However, Kerala Chief Minister Pinarayi Vijayan said the two new cases in his state -- two foreign returnees who had reached Kerala on 7 May in two separate flights under a massive ongoing evacuation plan of the Central Government -- is a warning for all states to be on an alert to strengthen their "mitigation efforts and preventive measures."
Many more similar flights from abroad are reaching Kerala and several other states over the next few days under what is being called the 'Vande Bharat' mission.
Besides the arrival of a large number of Indians stranded abroad, experts have also warned that the numbers may rise further in the coming days due to the ongoing movement of lakhs of migrant workers being facilitated by trains and buses to help them reach their native places.
On Saturday, the situation remained worrying in India's major hotspots like Mumbai, Pune, Delhi, Ahmedabad and Chennai, among others. While Mumbai cases reached 12,864 cases, with the metropolis now accounting for more than of Maharashtra's total infections, the adjoining Thane district crossed the 2000-mark.
In Chennai, the number of cases linked to the Koyambedu market cluster reached 1,867 with the market for vegetables, flowers and fruits eclipsing the Tablighi Jamaat event in Delhi (in which about 1,500 people from Tamil Nadu had taken part in March) for the dispersion of the virus.
Besides, reports of fresh infections in the Central Armed Police Forces, which is responsible for providing security along the border as well as securing key industries and establishments, presenting a fresh challenge for the Central Government.
Goa, on the other hand, which is classified as a green zone with no coronavirus positive case as of now, hinted that it may allow holding of music classes and reopening of some state-run libraries in a phased manner on the condition of maintaining strict social distancing norms.
Cases from states: Maharashtra crosses 20,000 mark
On Saturday, Maharashtra reported 1,165 new coronavirus patients, taking the tally of COVID-19 cases in the state to 20,228. The state also reported death of 48 patients, taking the death toll to 779, said a health department official.
Among those died of COVID-19 on Saturday was a 51-year-old police constable from Maharashtra's Nashik district, an official said.
This is the sixth death of a police personnel due to COVID-19 in the state, the official added.
Meanwhile, in Gujarat, the number of coronavirus cases in Gujarat climbed to 7,797 on Saturday with 394 new cases coming to light, while death toll rose to 472 with 23 more deaths, a senior health official said.
Of the total new cases, 280 were reported from hotspot Ahmedabad, along with 20 more deaths on Saturday, taking the total case count to 5,540 in the district and fatalities to 363, a health department official said.
In Gujarat, the number of patients who died in 24 hours since Friday evening — 23 — was the lowest in the last seven days, Principal Secretary (Health) Jayanti Ravi said.
Four COVID-19 deaths were reported in Tamil Nadu on Saturday with the state recording 526 more positive cases, including a five day old baby, taking the total number to 6,535, the health department said.
The deceased were all women, with three hailing from Chennai and one from Ramanathapuram. With this, the death toll has gone up to 44, a health department bulletin said.
Of the total of 526 positive cases, Chennai continued to lead the numbers with 279, followed by Villupuram 67 and Chengalpattu at 40.
In the National Capital Delhi also, 224 new COVID-19 cases were recorded to take its tally to 6,542.
In Delhi, there was also confusion over the death toll as the data from the four hospitals showed more fatalities than the number reported by the Delhi government. Delhi health minister Satyendar Jain said there is no reason to hide anything and not a single case will go unaccounted for, but gave no explanation for the discrepancy.
There has been a mismatch in the numbers of West Bengal also for several days with the state government's figures being lower than that of the Union Health Ministry.
On the outskirts of the National Capital, Noida reported its second COVID-19 death while its total cases rose to 216. The overall figure for Uttar Pradesh also rose.
Rajasthan recorded 76 more cases, while 36 new cases were detected in Karnataka too.
In Bihar, five Bihar Military Police (BMP) personnel tested positive, taking the total number of COVID-19 cases in the state to 579. In Assam, a dental college student's test came positive.
Central forces report 116 new cases
The growing number of infections in five Central Armed Police Forces (CAPFs) - CRPF, BSF, ITBP, CISF and SSB - under the command of the Union home ministry, has emerged as another challenge for the Central Government.
On Saturday, at least 116 fresh coronavirus infections were reported by the uniformed organisation, taking the total number of positive personnel to over 650, officials said.
Of the new cases, CRPF accounts for 62, BSF 35, CISF 13 and ITBP reported 6 fresh cases. As of now, the total active cases in these forces stands at 231 in CRPF, 256 in BSF, 48 in CISF and 100 in the ITBP.
Five personnel of these forces have succumbed to the disease, a senior paramilitary officer said.
After home minister Shah's review, the AIIMS in Jhajjar has been designated as a special coronavirus facility for the CAPF personnel apart from their 200-bedded referral hospital in Greater Noida.
Health ministry revises policy for discharge
The Union Health Ministry has also revised its policy for discharge of COVID-19 patients under which only those developing severe illness or having compromised immunity will have to test negative through RT-PCR test before being discharged by a hospital.
Moderate cases of COVID-19 and pre-symptomatic, mild and very mild cases need not undergo tests before being discharged after resolution of their symptoms.
According to the rules till now, a patient was considered fit to be discharged if he or she tested negative on day 14 and then again in a span of 24 hours.
Political tussle over migrant trains continue
The bodies of sixteen migrant labourers who were mown down by a goods train in Maharashtra's Aurangabad district were brought to Jabalpur by two bogies attached to a special train.
From Jabalpur, the coaches were further sent to Shahdol and Umaria, said a police officer.
Karnataka, which has been at the centre of a controversy over the state government disallowing migrants to leave the state, clarified on Saturday that migrant workers, pilgrims, tourists, students and other persons can hire and use buses provided by state-run road transport corporations on payment basis for travel to other states with relevant permissions.
However, the political tussle over special trains for stranded migrant lbourers continued on Saturday with Union home minister Amit Shah, in a letter to Chief Minister Mamata Banerjee alleged that the state was not allowing trains with migrant workers reach the state and termed it as an "injustice" towards these workers.
The state government, however, dismissed the charge by saying 6,000 stranded workers have already been brought back, and the state has given green signal to 10 trains carrying more such workers.
This claim was rejected by Indian Railways with officials too saying that there was no proposal on record so far with the national transporter to run any more 'shramik' trains to the state. According to reports, so far only two trains ferrying migrants have reached West Bengal.
The railways, however, on Saturday night said it had received "clearance" from West Bengal for running eight special trains to the state to ferry people stranded outside due to the ongoing lockdown.
On Saturday, a war of words also erupted between the JD(U) and the AAP over train fares of 1,200 Bihar-bound migrant workers, with the Arvind Kejriwal dispensation saying that it bore the cost of ferrying the migrants, a claim rejected by the JD(U) which said the party was speaking half-truth on the issue.
The ruling Janata Dal (United) in Bihar said the AAP-led Delhi government has sought reimbursement of the payment and accused the Aam Aadmi Party (AAP) of resorting to "cheap politics to gain popularity".
Meanwhile, NCP chief Sharad Pawaron Saturday urged Prime Minister Narendra Modi to speak to the chief ministers of states, who are not allowing migrants to return home.
"I humbly request our @PMOIndia Shri Narendra Modi ji to intervene in this matter by talking to the CMs of the respective states who are not allowing these people to come back home," Pawar said in a series of tweets without mentioning any specific state.
362 people from West Asia arrive in Kochi
As for Indians stranded abroad, two Air India Express flights carrying 362 people from Oman and Kuwait arrived at the international airport in Kochi on Saturday night.
There were a total of 362 people, including eight infants in the flights which landed here from the two Gulf nations, a Cochin International Airport Limited (CIAL) spokesman said.
Official sources said all the passengers would be subjected to COVID-19 rapid test at the airport before being transferred to their respective destinations by special taxis and KSRTC buses.
They will go into quarantine after completing formalities at the airport.
Another Air India Express flight from Doha, carrying 177 passengers and six infants, is expected to arrive here early hours on Sunday, sources said.
Another special Air India flight carrying 177 Indians has left Kula Lumpur for Trichy, Tamil Nadu, ANI said.
According to sources, three other Air India flights - from Singapore (with 243 passengers), from London with (329 passengers) and from Manila (241 passengers) are expected to reach Mumbai on Sunday.
Globally, nearly 40 lakh people have tested positive for the deadly virus since its emergence in China last December, while approximately 2.75 lakh people have lost their lives. However, nearly 13 lakh people have recovered too, including nearly 2 lakh in the US and over 1.4 lakh in Germany.
Germany and South Korea are among those countries that have been seen as having successfully avoided large number of deaths by their extensive testing and contact tracing measures. But, worries mounted on Saturday about fresh outbreaks in both the countries following various lockdown relaxations, thus raising the risks associated with reopening of economies.
With inputs from agencies
http://sansaartimes.blogspot.com/2020/05/coronavirus-outbreak-live-updates-first.html
1 note
·
View note
Text
COVID-19 | Alert, Information
COVID-19 | Alert, Information & Advisory
For the past One month, we frequently heard a name Novel Coronavirus or COVID-19. Almost the world under lockdown. Is COVID-19 is that much Serious Disease? Why this much serious shown towards Coronavirus? What is Novel Coronavirus Disease? What are the symptoms of COVID-19? What should I do if i found myself as a COIVD-19 Positive? How to prevent us from this Corona Virus? Let’s See Deeply on this article.

Stay Home, Stay Safe…! This is the slogan we heard from every direction. Every World leaders start their speech with this slogan from the past one-month Lets enter into the article and we get clear answer for all the above questions.
What is Novel Coronavirus Disease?
Coronaviruses are a large family of viruses that may cause illness in animals or humans. In humans, several coronaviruses are known to cause respiratory infections ranging from the common cold to more severe diseases such as Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS). The most recently discovered coronavirus causes coronavirus disease COVID-19.
What is COVID-19
COVID-19 is an infectious disease caused by the most recently discovered coronavirus. This new virus and disease were unknown before the outbreak began in Wuhan, China, in December 2019.
What are the symptoms of COVID-19?
The COVID-19 virus affects different people in different ways. COVID-19 is a respiratory disease and most infected people will develop mild to moderate symptoms and recover without requiring special treatment. People who have underlying medical conditions and those over 60 years old have a higher risk of developing severe disease and death.
Common symptoms include:
fever
tiredness
dry cough.
Other symptoms include:
shortness of breath
aches and pains
sore throat
and very few people will report diarrhea, nausea or a runny nose.
People with mild symptoms who are otherwise healthy should self-isolate and contact their medical provider or a COVID-19 information line for advice on testing and referral.
People with fever, cough or difficulty breathing should call their doctor and seek medical attention.
What should I do if i found myself as a COIVD-19 Positive?
First, Don’t panic. It is a 100% not deadly disease if you have immunity in your body to fight for the next 14 days you will be 100% cured. As of now, the death rate is only 3-5% of the total affected.
Panic is the first enemy to fight against COVID-19. Based on research fear is decreasing your immunity power. So don’t get panic first, You just isolate yourself from others and inform your doctor or help-care that you having those above symptoms. In case if your test result is positive don’t worry you have a 95% chance get recovered completely from this disease.
At the time of writing this article there was no exact medicine was found against coronavirus. You can ask me then how you get to recover from coronavirus without and antibiotic. Yes your white cells take charge here. your inborn immunity power will kill coronavirus. But it takes a couple of weeks. for that time you should be alive that’s all.
For that,
keep your room or living area clean to low down the virus density around you.
Get Self-isolated to avoid spreading
Take High immunity foods like Tea, Raw Citrus drinks, Mint, Pepper, Ginger water.
Avoid carbonated drinks and fried items.
I will suggest some supplements to improve your immunity at the last of this article. Non-affected peoples can also take those supplements which is FDA approved.
How does COVID-19 spread
People can catch COVID-19 from others who have the virus. The disease can spread from person to person through small droplets from the nose or mouth which are spread when a person with COVID-19 coughs or exhales. These droplets land on objects and surfaces around the person. Other people then catch COVID-19 by touching these objects or surfaces, then touching their eyes, nose or mouth. People can also catch COVID-19 if they breathe in droplets from a person with COVID-19 who coughs out or exhales droplets. This is why it is important to stay more than 1 meter (3 feet) away from a person who is sick.
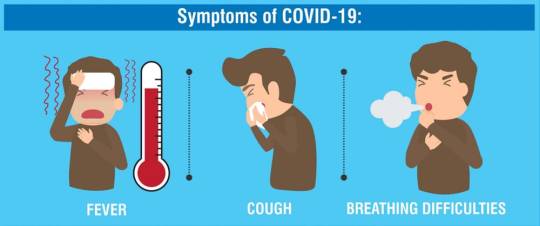
Can the virus that causes COVID-19 be transmitted through the air?
Studies to date suggest that the virus that causes COVID-19 is mainly transmitted through contact with respiratory droplets rather than through the air. See previous answer on “How does COVID-19 spread?”
Can CoVID-19 be caught from a person who has no symptoms?
The main way the disease spreads is through respiratory droplets expelled by someone who is coughing. The risk of catching COVID-19 from someone with no symptoms at all is very low. However, many people with COVID-19 experience only mild symptoms. This is particularly true at the early stages of the disease. It is, therefore, possible to catch COVID-19 from someone who has, for example, just a mild cough and does not feel ill.
Can I catch COVID-19 from the feces of someone with the disease?
The risk of catching COVID-19 from the feces of an infected person appears to below. While initial investigations suggest the virus may be present in feces in some cases, spread through this route is not a main feature of the outbreak. The ongoing research on the ways COVID-19 is spread and will continue to share new findings. Because this is a risk, however, it is another reason to clean hands regularly, after using the bathroom and before eating.
Final Words
Our world had more experience in the past like this pandemic disease. So don’t worry first CoVID-19 is not a killing virus but it’s a fast-spreading virus. So only all governments take severe action against this disease. If the number of the patient increased the only difficulty to the government providing ventilation for all. Apart from that prevention is better than cure. So keep self-isolated for a couple of months. Keep your self-sanitary things and self-hygiene Keep your environment as humility free. Stay home… Stay Safe…!
1 note
·
View note
Text
'Promising' breathalyzer-style COVID test highlights need for better data, experts say
‘Promising’ breathalyzer-style COVID test highlights need for better data, experts say
New ways of testing for COVID-19 bring promises of accessibility and fast results, but that doesn’t diminish the need for consistent national data on case counts, experts say.
As Canada loses track of case counts, a variety of new COVID testing technologies are emerging across North America. In mid-April, the U.S Food and Drug Administration (FDA) approved the first breath test for the virus,…

View On WordPress
0 notes