#sulphonamide
Photo
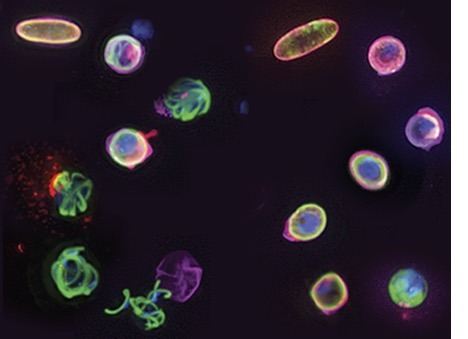
Harmless Immaturity
Nip it in the bud and break the cycle. That’s the goal of a newly-discovered class of malaria treatments, which aim to interrupt the deadly parasite’s life cycle and prevent its spread. But how exactly the compounds, called sulphonamides, work is unclear. So researchers added a light-activated marker to the drug, and observed as it interacted with infected human blood cells. The marker then highlighted which parasite proteins the treatment interacted with, in particular a protein called Pfs16. With the drug bound to Pfs16, part of the parasite’s maturation process was blocked. The parasites couldn't make mature male sex cells, rendering them unable to reproduce and spread (pictured): green treated parasites (right) unable to 'fly the nest' while untreated, left, break free from the red human cells. Understanding the timing and mechanisms of this process is key to converting this discovery into practical treatments to end transmission for good.
Written by Anthony Lewis
Image adapted from work by Sabrina Yahiya and colleagues
Department of Life Sciences, Imperial College London, Sir Alexander Fleming Building, Exhibition Road, South Kensington, London, UK
Image originally published with a Creative Commons Attribution 4.0 International (CC BY 4.0)
Published in Disease Models & Mechanisms, January 2023
You can also follow BPoD on Instagram, Twitter and Facebook
#science#biomedicine#malaria#parasites#plasmodium#immunofluorescence#anti-malarials#sulphonamide#red blood cells
8 notes
·
View notes
Text
Sexy BIG TITTED chick grabbed and FUCKED HARDCORE on wooden desk
SoapyMassage Asa Akira Gives Lesbian First Massage
High school sluts jizzed
Young angelic latin babe babe gets her petite titts played hard
Gianna Michaels Is Down For Some Kinky Public Fucking
Pussy eating tiny lesbian
What Every Frenchwoman Wants Watch Online full Movie
Huge Boobs Fat Ebony Olivia Leigh Goes to Town on a White Dick
Horny stepmom licks her stepteens pussy
Pinay wife riding Indian dick
#gemological#trinunity#sulphonamide#manned#madre#preamplifiers#polycotyledonous#budged#fastidium#preshowing#marabotin#Denizlik#pulicosity#unfeudal#antecurvature#two-handedness#arterio-#armaria#respiratored#walkist
1 note
·
View note
Link
0 notes
Text
#felt obligatory ngl#chemistry#cbse#jee#neet#amines#sample papers#questions#polls#tumblr polls#academic polls#vanilla#vanilla extract
24 notes
·
View notes
Text
Major Ferguson was saying. “The fractured ends of the femur were extensively exposed and penetrated with gravel and so on. The osteomyelitis responded remarkably well to sulphonamides, but, as you see, we had to open four times in all to remove various sequestra, and about a month ago we began to feel he’d probably be better off without it. However, the callus started to look more promising, and the question then was whether amputation would be justified by the increased mobility he’d get from an artificial limb.”
“The knee’s completely ankylosed, is it?” The brigadier sounded like an intelligent player discussing a chess problem.
“No, sir, we managed to give him a flexion of about twenty degrees, and that decided us to leave it, combined with the fact that we’ve reduced the shortening to just about an inch. The repair of the quadriceps …”
Poor Laurie :( Hearing all this speech and in addition having the person who was flirting with early, listening and looking at his wound... A traumatic and embarrass experience, indeed
3 notes
·
View notes
Text
“Ivy Roe”, 31 (UK 1971)
A study published in a 1971 edition of the British Medical Journal reports the painful death of a 31-year-old woman who underwent a “safe and legal” abortion at a hospital. She was later given the name Ivy Roe.

Abortion on demand was illegal in the UK until the 1967 Abortion Act, which allowed them to be done up to 28 weeks (later reduced to 24 weeks in 1990) and sometimes later. After abortion was legalized, the West Middlesex Hospital began conducting “safe and legal” abortions with various methods. The data on the side effects to the clients was used to determine the risk of the different methods.
Out of all the West Middlesex Hospital abortion clients undergoing “safe and legal” abortions with experimental techniques, 83 underwent intrauterine insertion of a substance referred to as utus paste. Contrary to an earlier claim from abortion supporters that this method had no serious complications, the clients suffered severe fever, UTI, retained fetal tissue, abnormal bleeding, cervical trauma, suspected peritonitis, tachycardia, sepsis, uterine perforation and death.
One of the 83 clients was 31-year-old Ivy Roe. The study notes that she was married and was 16 weeks pregnant. Before the abortion, Ivy was in good condition. It is unclear if Ivy knew that she was being tested on and she may not have ever consented to being part of an experiment.
Ivy went into contractions and premature labor as planned. 20 hours after the abortion was started, she had her dead baby. 4 hours after that, her uterus was dilated and curettage was used to remove any body parts or placenta that might have been left behind. This was the procedure for all of the 83 test subjects whose abortions were considered successful at that point.
By the next day, Ivy was already showing signs that something was seriously wrong. She had a fever and her uterus was swollen to the size that it would have been if she were 12 weeks pregnant. She was given antibiotics for 3 days and then sent home even though her uterus was still swollen.
When she was home, Ivy was still in pain. She had to urinate far too frequently, and suffered abdominal pain. She went to a doctor, who noticed her tachycardia but didn’t find a fever and gave her sulphonamides and antibiotics.
A week after being discharged from West Middlesex Hospital, Ivy was dead.
Ivy’s autopsy was sickening and horrifying. Her uterus was ruptured and had necrotic tissue. The uterine walls were congested and there was thick green pus. She died a slow and excruciating death from her severe infection and internal injuries.
Ivy was promised a “safe and legal” abortion, but she didn’t know that legalization didn’t make it safe.
#death from legal abortion#tw abortion#unsafe yet legal#pro life#abortion#abortion debate#tw ab*rtion#pro choice#tw murder#tw human experimentation
4 notes
·
View notes
Text
Global Focus on Improved Outcomes: Global Veterinary Antibiotics Market
The global veterinary antibiotics market is projected to have a value of US$11,453.3 million in 2023. By 2033, the veterinary antibiotics market is projected to have grown at a slow 5.8% CAGR to reach US$ 20,154.0 million.
The veterinary antibiotics market is rising due to the growing animal healthcare sector and continuous innovation in the sector. Based on product type, tetracycline is expected to hold a dominant market share in the global demand for veterinary antibiotics. This is a result of its extensive use in animals raised for food.
Preview Next-Level Insights Sample :
https://www.futuremarketinsights.com/reports/sample/rep-gb-4295
Innovation and Expanding Animal Care Fuel Market Growth
The veterinary antibiotics market is poised for consistent growth, reaching an estimated value of US$20.2 billion by 2033. This growth is attributed to the continuous innovation within the animal healthcare industry and the increasing emphasis on pet health.
Tetracycline Reigns Supreme in Food Animal Production
Tetracycline antibiotics are expected to maintain their leading position within the veterinary antibiotics market. Their broad-spectrum effectiveness makes them a popular choice for treating infections in animals raised for food production.
Rising Pet Ownership and Zoonotic Disease Concerns Drive Demand
The growing number of pet owners worldwide is leading to a heightened awareness of pet health. Additionally, the increasing concern about zoonotic diseases, which can be transmitted from animals to humans, is driving the demand for effective veterinary antibiotics.
Key Takeaways:
The global veterinary antibiotics market is expected to reach US$20,154.0 million by 2033, reflecting a steady growth at a CAGR of 5.8%.
The expanding animal healthcare industry, rising pet ownership, and increasing awareness of zoonotic diseases are key drivers for market growth.
The dominance of tetracycline antibiotics used in food production animals is anticipated to continue.
How Key Players are Contributing to the Veterinary Antibiotics Market:
The veterinary antibiotics market is seeing an increase in collaboration between players. The primary reason is that collaborations provide companies access to a greater range of products and a more diverse technology set. Antibiotics generated as a result of this cooperation are of higher quality and manufactured faster.
Recent Developments Observed by FMI:
The ‘Nandi’ Portal was introduced in June 2023 to enable online approval of new veterinary drugs and vaccines in India. Nandi is an acronym for New Drug and Immunization Device Approval. As the entire form indicates, Nandi intends to make it easier for novel veterinary medications and vaccinations to receive NOCs.
For the Indian Pharmacopoeia (IP) 2022, the Indian Pharmacopoeia Commission (IPC) introduced seven new veterinary monographs and eight new chemical monographs with the intention of boosting both public and animal health in the nation. Carprofen pills, Pimobendan capsules, Selamectin, Tilmicosin, Tilmicosin injectable, and Triclabendazole are among the recently released veterinary monographs.
A partnership between the Pride Veterinary Medical Community and the United States-based animal health technology business Covetous was announced in June of 2021. Covetrus is situated in the United States. With the help of this partnership, Covetrus can offer cutting-edge veterinary medicine, which in turn empowers veterinary healthcare professionals.
Adiva GmbH and Bayer AG partnered to produce therapeutic antibodies for veterinary medicine in April 2019.
Key Players in the Global Market:
Zoetis Inc.
Merck & Co.
Bayer AG
Sanofi
Eli Lilly and Company
Ceva Sante Animale
Others
Veterinary Antibiotics Market Segmentation:
By Product Type:
Tetracyclines
Penicillins
Macrolides
Sulphonamides
Aminoglycosides
Others
By Route of Administration:
Premixes
Injections
Oral Powders
Oral Solutions
Others
By Animal Type:
Food-processing Animals
Companion Animals
By Region:
North America
Latin America
Europe
Asia Pacific
The Middle East & Africa (MEA)
0 notes
Text
Folitrax 10mg: Everything You Need to Know About This Medication
About Folitrax 10 Tablets 10
Folitrax-10 Tablet belongs to the antimetabolite group of drugs. It is used in the treatment of various types of arthritis such as rheumatoid arthritis, psoriatic arthritis, reactive arthritis, vasculitis, enteropathic arthritis, myositis, and systemic sclerosis.
It can also be given to children who have juvenile idiopathic arthritis, systemic lupus erythematosus, juvenile dermatomyositis, vasculitis, uveitis, and localised scleroderma.
Inform your doctor about any pre-existing diseases and the medicines you are taking. Your doctor will carefully review and decide regarding the dosage and frequency of use of this medicine.
Uses of Folitrax 10 Tablets 10
Folitrax-10 Tablet is used in the treatment of:
Various types of arthritis such as rheumatoid arthritis, psoriatic arthritis, reactive arthritis, vasculitis, enteropathic arthritis, myositis, and systemic sclerosis
In children who have juvenile idiopathic arthritis, systemic lupus erythematosus, juvenile dermatomyositis, vasculitis, uveitis, and localised scleroderma
Directions for Use of Folitrax 10 Tablets 10
Take the tablet with whole glass of water.
Swallow the tablet; do not crush or chew it.
Take the tablet at fixed times each day to maintain a consistent level of medication.
Side Effects of Folitrax 10 Tablets 10
The common side effects of Folitrax-10 Tablet are
Headache
Vomiting
Diarrhoea
Dizziness
Nausea
Managing the Side Effects:
Most side effects are temporary and generally harmless, which resolve on discontinuing Folitrax-10 Tablet. However, if you experience any serious side effects or worsening of any of the symptoms, please consult your doctor.
How Folitrax 10 Tablets 10 Works?
Folitrax-10 Tablet contains the following component:
Methotrexate (10 mg): It is an antimetabolite drug that exerts its therapeutic effects by inhibiting dihydrofolate reductase, an enzyme essential for folic acid metabolism. By disrupting this pathway, methotrexate hampers the synthesis of DNA, RNA, and proteins, which are crucial components for cell replication. Consequently, it suppresses the abnormal immune response responsible for inflammation in conditions like rheumatoid arthritis. Methotrexate's immunosuppressive and anti-inflammatory actions contribute to its effectiveness in managing autoimmune diseases, making it a cornerstone in the treatment of various inflammatory conditions.
Interactions of Folitrax 10 Tablets 10
Drug-Drug Interactions
Folitrax-10 Tablet can interact with medicines, including
Salicylates like aspirin, when taken with this tablet, can increase the effect of this tablet.
Antibiotics like chloramphenicol, penicillin, sulphonamides, and co-trimoxazole may cause methotrexate toxicity when taken togehter.
Diuretics like hydrochlorothiazide may interact with this tablet and cause kidney issues when taken together.
Epilepsy medicines like phenytoin, when taken with this tablet, can increase the risk of seizures.
Gout medicines like probenecid, when taken together with this tablet, can increase the blood levels of this tablet.
Vitamin preparations like folic acid, when taken together with this tablet, can decrease the the efficacy of this tablet.
Antacid like omeprazole and omeprazole-like medicines that control stomach acid may interact and increase levels this tablet.
Anti-cancer agents like cisplatin, mercaptopurine can cause nephrotoxicity and ototoxicity when taken with this tablet.
Non-steroidal anti-inflammatory medicines like ibuprofen may increase the levels of this tablet inside the body.
Rheumatism control drugs like azathioprine can cause toxicity when taken with this medicine.
Medicines used to treat respiratory diseases, like theophylline can have increased levels when taken with tablet.
Immuno-suppressive medicines like cyclosporine can have increased levels when taken with this medicine.
Drug-Disease Interactions
Folitrax-10 Tablet can interact with medicines, including
Liver diseases: This tablet can worsen the liver diseases.
Kidney diseases: This tablet can worsen the kidney diseases.
Peptic ulcer/ ulcerative colitis: This tablet can worsen this condition.
Vaccines: The concomitant use of this tablet with vaccines can affect the methotrexate levels.
Immunodeficiency: This tablet can further worsen the immunodeficiency.
Bone marrow disease: This tablet can cause bone marrow suppression.
Serious blood disorders: This tablet can lower the number of white blood cells leading to risk of increased infection.
Dosage of Folitrax 10 Tablets 10
Daily Dose
The recommended daily dose of Folitrax-10 Tablet should be taken as prescribed by your doctor. The dosage may change based on individual factors such as age, severity of the condition, and response to treatment.
Over Dose
If an excessive dosage of Folitrax-10 Tablet is taken by accident; taking immediate medical attention from your doctor is essential in case you experience any severe side effects or worsening of any of the symptoms.
Missed Dose
If you forget to take a Folitrax-10 Tablet, take it as soon as possible. However, avoid repeating the dose.
Storage
Tablet should be stored below 30°Celsius.
Store this tablet away from children’s reach.
Do not use this tablet once the expiry date has passed.
Protect the medicine from excessive light and moisture.
0 notes
Text
How Do Antibiotics Work?
Antibiotics are specialized medicines designed to combat infections caused by bacteria. The term “antibiotic” signifies “against life.” Antibiotic medications are commonly used for treating and preventing infections. Two classes of antibiotics are employed to halt bacterial growth: bacteriostatic and bactericidal. The primary distinction lies in the method of action. Bactericidal antibiotics cause direct bacterial death by preventing the production of bacterial cell walls, leading to an irreversible effect. Examples include beta-lactam antibiotics, cephalosporins, and vancomycin. On the other hand, bacteriostatic antibiotics prevent bacterial DNA replication and protein synthesis, with reversible effects. Examples include tetracyclines, spectinomycin, chloramphenicol, sulphonamides, trimethoprim, lacosamides, and macrolides. At high concentrations, bacteriostatic antibiotics may also exhibit bactericidal effects.
These drugs are available in various forms, such as tablets, capsules, liquids, creams, and ointments. While many antibiotics require a prescription, certain creams and ointments are available over the counter. Their primary function is to eliminate or hinder the growth of bacteria in the body. Antibiotics combat bacterial infections by attacking the bacterial wall, interfering with reproduction, or blocking protein production.
Bacterial cells typically consist of a cell wall, cell membrane, and nucleus. The cell wall, an outer layer made up of peptidoglycan with cross-linked polymers, is crucial for resistance mechanisms and virulence factors, shaping the bacteria. Multiple layers of peptidoglycan, composed of glycans and peptide chains, form the bacterial cell wall. N-acetyl glucosamine and n-acetyl muramic acid combine to form the cell wall glycans, facilitated by transglycosidases. In the presence of penicillin-binding proteins (PBPs), glycine residues cross-link the d-alanyl-d-alanine section of the peptide chain. The bacterial cell wall can be likened to a hard outer layer composed of linked protein and sugar blocks, essential for the bacteria’s shape, strength, and resistance to hazardous substances.
β-lactam antibiotics, including penicillins and cephalosporins, commonly function by preventing the formation of bacterial cell walls. The main focus of β-lactam agents is on penicillin-binding proteins (PBPs). The β-lactam ring in these antibiotics mimics the d-alanyl d-alanine segment, a typical binding site for PBPs. Interaction with the β-lactam ring prevents PBPs from participating in new peptidoglycan synthesis, ultimately causing the breakdown of the peptidoglycan layer and bacterial lysis.
Folic acid is essential for DNA and RNA synthesis, as well as for the processes of growth and multiplication. Humans obtain folic acid from the diet, while bacteria need to produce their own. Trimethoprim and sulphonamides inhibit different stages of folic acid production. Sulphonamide binds to the enzyme dihydropteroate synthase, preventing the conversion of para-aminobenzoic acid (PABA) into dihydrofolate (DHF). Trimethoprim inhibits the enzyme dihydrofolate reductase, necessary for producing tetrahydrofolate (THF). Together, trimethoprim and sulphonamide work synergistically to lower the rate of resistance mutation development.
Antibiotics that block protein synthesis typically target bacterial ribosomes, essential for protein synthesis. Bacterial ribosomes have two asymmetrical subunits, 30S and 50S. Aminoglycosides like gentamicin and streptomycin bind to the 30S subunit, preventing the formation of the initiation complex. This binding causes mRNA misreading and the addition of incorrect amino acids to the growing polypeptide chain, leading to bacterial cell death by releasing toxic or nonfunctional proteins. Understanding these mechanisms of action is crucial for selecting the right antibiotics for specific infections and avoiding resistance development.

Name: Vrushali Shantaram Dongare
Class: M. Pharm (Sem- III)
Department: Pharmacology
More details- https://aissmscop.com/how-do-antibiotics-work/
0 notes
Text
Mueller Hinton Agar: The Media for the Kirby-Bauer Method
Mueller Hinton Agar (MHA) is a solid medium used for the routine susceptibility testing of non-fastidious microorganisms. It was developed by Mueller and Hinton in 1941 for the isolation of Neisseria species. MHA is non-selective and non-differential, which makes it suitable for the growth of any organism. Mueller Hinton Agar acts as a standardized medium for the Kirby-Bauer disc diffusion method, a widely used technique for determining antibiotic susceptibility. This method involves placing antibiotic-soaked discs on the agar surface, allowing them to diffuse into the surrounding medium.

Kirby-Bauer susceptibility testing with MHA
MHA is a multipurpose culture media that allows the growth of all non-fastidious bacteria. The presence of starch in the media absorbs all the toxins released by the bacteria, which could interfere with the antibiotics. MHA is a loose agar plate, thus increasing the rate of antibiotic diffusion and providing a clear depiction of the zone of inhibition. Batch-to-batch reproducibility is a major factor in adopting this media for antibiotic susceptibility tests for microbes. The concentration of inhibitors is very low in the medium, which reduces the inactivation of sulphonamides and trimethoprim during the susceptibility of bacteria to antimicrobials. The interpretation of the results obtained from MHA involves measuring the diameter of the clear zones around the antibiotic discs. Larger zones indicate greater susceptibility of the bacteria to the antibiotic, while smaller or absent zones suggest resistance.
Composition of MHA
Ingredients (Gms/ltr)
Agar 17.000
Casein acid hydrolysate 17.500
Beef, infusion 2.000
Starch 1.500
TM Media’s MHA ensures consistency and reproduces exact results. It consists of beef extract, casein hydrolysate, and starch. Beef extract and the acid hydrolysate of casein provide all the essential nutrients for growth, like nitrogen, vitamin, carbon, amino acids, sulphur, etc. Starch plays a characteristic role in absorbing toxic substances released by bacteria during growth. Agar is the solidifying agent.
TM Media’s Mueller Hinton Agar
TM Media provides Mueller Hinton Agar in two forms: Dehydrated and Ready-to-Use, fulfilling the various needs of the microbiologist. The Ready-to-Use MHA Plates reduce any manual error with higher accuracy and productivity as they are ready-to-use and pre-sterilized. They also provide antibiotic discs for susceptibility testing along with all the necessary laboratory equipment, like Petri Plates, Nichrome Loops, Disposable Swabs and many more.
Summary
In conclusion, Mueller-Hinton Agar is a fundamental component in the field of microbiology, particularly in antibiotic susceptibility testing. Its standardized composition and reliable performance make it an invaluable tool for assessing the efficacy of antibiotics and guiding clinicians in the selection of appropriate treatments for bacterial infections.
#Mueller Hinton Agar#dehydrated culture media#antibiotic susceptibility#Kirby-Bauer#Mueller and Hinton
0 notes
Text
Mueller Hinton Agar
Mueller Hinton Agar, developed by John Howard Mueller and Jane Hinton in 1941, is a simple and transparent agar medium that was originally formulated for the cultivation of Neisseria species. Later, it became widely used for the determination of sulphonamide resistance in gonococci and other organisms.
It is a non-selective and non-differential culture media which is widely used for the Kirby-Bauer method of Antimicrobial Susceptibility Testing. It is approved and recommended by the Clinical and Laboratory Standards Institute (CLSI) because of its consistent results, its low presence in sulphonamide, trimethoprim, and tetracycline inhibitors, and its ability to support the growth of most non-fastidious pathogenic bacteria.
Composition of Muller Hinton Agar:
Mueller Hinton Agar is composed of beef infusion, casein acid hydrolysate, starch, and agar. Beef infusion and casein acid hydrolysate supply nitrogenous compounds, carbon, sulphur, and other essential nutrients. Starch acts as a dispersing medium and absorbs any toxic metabolites if they are produced. Starch hydrolysis produces dextrose, which functions as an energy source.

Preparation of Mueller Hinton Agar:
To prepare Mueller Hinton Agar, liquefy 38 grams of the medium in 1 litre of purified or distilled water.
Heat the mixture to boiling to dissolve the medium completely.
Sterilize the medium by autoclaving at 15 psi pressure (121°C) for 15 minutes.
Allow the medium to cool to 45-50°C, then mix well and pour it into sterile Petri dishes.
The manual preparation of Media is a time consuming process. Hence, to save time, resources, and energy, Ready-to-Use Plates are the best option. TM Media offers a wide range of Ready-to-Use Plates tailored for specific applications.
TM Media’s Muller Hinton Agar Plate
TM Media’s Ready-to-Use Mueller Agar Plate serves convenience, consistency, and reliability and also saves preparatory time and effort. Additionally, it is approved and certified by all the strict industry standards.
TM Media’s Mueller Hinton Agar (Dehydrated Culture Media) offers two pack sizes- 100 gm and 500 gm.

Conclusion:
In the world of microbiology, Mueller Hinton Agar has proven to be a significant tool. Its composition, neutral pH, and consistent performance make it an essential medium for reliable antimicrobial susceptibility testing.
TM Media is one of the finest manufacturers of Culture Media. TM Media has over 4000 products in different categories - Dehydrated Culture Media, Ready-to-Use Culture Media, Biological Media Bases, Media Supplements, Indicators, Laboratory Chemicals, Antibiotic Sensitivity Discs, and many more.
To know more about TM Media and our Antibiotic Sensitivity Discs, read this blog.
#mueller hinton agar#dehydrated culture meedia#antibiotic sensitivity discs#antimicrobial susceptibility testing#AST#microbiology#ready to use culture media#ready to use plates
0 notes
Text
Sulphonamides Cotrimoxazole And Quinolones Question And Answers
#Pharmacology#PharmaceuticalIndustry#ClinicalPharmacology#DrugsandResearch#sulphonamidescotrimoxazoleandquinolonesquestionandanswers
0 notes
Text
Sulphonamides Cotrimoxazole And Quinolones Question And Answers
#Pharmacology#PharmaceuticalIndustry#ClinicalPharmacology#DrugsandResearch#sulphonamidescotrimoxazoleandquinolonesquestionandanswers
0 notes
Text
0 notes
Text
Urinary Tract Infection (UTI) Treatment Market Report- Drivers and Restraints, Growth Opportunity Assessment and Forecast to 2032
The increasing prevalence of urinary tract infection (UTI) and growth in the number of prescription of quinolones antibiotics is expected to fuel the growth of the urinary tract infection treatment market. According to a new study published by Future Market Insights (FMI), the global urinary tract infection treatment market revenues will grow at a mere 2% CAGR between 2019 and 2029.
Launch of New Antibiotics for Bacterial Infections to Sustain Revenues
Increase in the number of research and development initiatives for the developing novel therapeutics has led to the launch of new drugs into the urinary tract infection treatment market. Manufacturers are currently focusing on expanding their pipeline portfolio for the treatment of bacterial infections. Moreover, regulatory bodies such as FDA have accelerated the approval process of drugs.
Request a Sample Report with Table of Contents and Figures: https://www.futuremarketinsights.com/reports/sample/rep-gb-1340
Recently, in August 2018, Melinta Therapeutics announced the launch of Vabomere (meropenem and vaborbactam) for patients who require treatment for complicated urinary tract infection. Similarly, one more drug has been approved by FDA in June 2018, Zemdri by Achaogen, Inc., for the treatment of adults suffering from Complicated Urinary Tract Infection (cUTI). Increase in the launch and approval of novel therapeutics for the treatment of bacterial infections is expected to contribute to the growth of the urinary tract infection treatment market.
Quinolones, the broad spectrum antibiotics effective against a wide range of bacterial disease, have bactericidal activity that enables them to penetrate deep into the tissue. Their excellent bioavailability is a plus point. In addition, they have favourable tolerability and safety profile. Moreover, they have significant antimicrobial activity. Thus, quinolones are most preferred for the treatment of urinary tract infection. Some of the widely used quinolones for the prevention of bacterial infection include ciprofloxacin (Cipro), ofloxacin (Floxin), norfloxacin (Noroxin), and others. Quinolones are mostly favored over trimethoprim-sulfamethoxazole (TMP-SMX) for the treatment of uncomplicated urinary tract infections owing to the increasing resistance of bacteria towards antibiotics such as trimethoprim-sulfamethoxazole, beta lactam antibiotics, and others
Talk with our analyst and get the complete information of report now@ https://www.futuremarketinsights.com/ask-question/rep-gb-1340
Europe Remains Major Revenue Contributor
Europe holds a considerable revenue share in the UTI treatment market. The rising prevalence of urinary tract infection among the population and increasing hospital visits are among factors that are likely to propel the growth of the urinary tract infection treatment market. It is estimated that the urinary tract infection treatment market will create an incremental $ opportunity worth nearly US$ 9 Mn between 2019 and 2029. Quinolones is anticipated to boost the growth of the urinary tract infection treatment market owing to the increasing prescription and high effectivity as antimicrobial antibiotics.
Demand from Retail Pharmacies to Register High Growth
The global urinary tract infection treatment market has been segmented on the basis of drug class, indication, and distribution channel. On the basis of drug class, the urinary tract infection treatment market has been segmented into Penicillin & Combinations, Quinolones, Cephalosporin, Aminoglycoside Antibiotics, Sulphonamides (Sulfamethoxazole +Trimethoprim), Azoles and Amphotericin B, Tetracycline (Doxycycline), Nitrofurans (Nitrofurantoin), and others. In terms of revenue, the quinolones segment is expected to hold a major share of the urinary tract infection treatment market during the forecast period since it is widely prescribed and has proven to be safe and effective for antimicrobial use.
0 notes
Text
Dorzolamide Hydrochloride CAS#:130693-82-2
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product NameDorzolamide HydrochlorideIUPAC Name(4S,6S)-4-(ethylamino)-6-methyl-7,7-dioxo-5,6-dihydro-4H-thienothiopyran-2-sulfonamide;hydrochloride Molecular StructureCAS Registry Number 130693-82-2EINECS Number620-304-2MDL NumberMFCD00884659Beilstein Registry Number5896026SynonymsDORZOLAMIDE HYDROCHLORIDE130693-82-2Dorzolamide HClTrusoptDorzolomide hydrochlorideCosoptDorzolamide (hydrochloride)MK-507Dorzolomide HCl(4S,6S)-4-(ethylamino)-6-methyl-5,6-dihydro-4H-thienothiopyran-2-sulfonamide 7,7-dioxide hydrochlorideDorzolamide (as hydrochloride)QZO5366EW7Trusopt (TN)CHEBI:4703(4S,6S)-4-(Ethylamino)-5,6-dihydro-6-methyl-4H-thieno(2,3-b)thiopyran-2-sulfonamide 7,7-dioxide, monohydrochloride(4S,6S)-4-(ethylamino)-6-methyl-7,7-dioxo-5,6-dihydro-4H-thienothiopyran-2-sulfonamide;hydrochloride4H-Thieno(2,3-b)thiopyran-2-sulfonamide, 4-(ethylamino)-5,6-dihydro-6-methyl-, 7,7-dioxide, monohydrochloride, (4S-trans)-UNII-QZO5366EW7SR-05000001449Dorzolamide hydrochloride (4S,6S)-4-(Ethylamino)-5,6-dihydro-6-methyl-4H-thienothiopyran-2-sulfonamide 7,7-dioxide, monohydrochlorideL 671152L-671,152Dorzolamide HCl saltDorzolamide hydrochloride MK507 hydrochlorideMK 0507MK-0507L671152 hydrochlorideNCGC00016977-01CAS-130693-82-2SCHEMBL41152MLS002154162CHEMBL1201162DTXSID1045530Dorzolamide hydrochloride- Bio-XHY-B0109AHMS1571O14EX-A3987Tox21_110720MFCD00884659s1375DORZOLAMIDE HYDROCHLORIDE AKOS005146235AKOS015895951Dorzolamide hydrochloride (JP17/USP)Tox21_110720_1AC-5244CCG-221116CS-1858DORZOLAMIDE HYDROCHLORIDE KS-1348DORZOLAMIDE HYDROCHLORIDE DORZOLAMIDE HYDROCHLORIDE NCGC00179244-03BD164381DORZOLAMIDE HYDROCHLORIDE DORZOLAMIDE HYDROCHLORIDE SMR001233461MK-507 (L-671152) HClD4189DORZOLAMIDE HYDROCHLORIDE, TRANS-(-)-C72221D00653DORZOLAMIDE HYDROCHLORIDE DORZOLAMIDE HYDROCHLORIDE COSOPT COMPONENT DORZOLAMIDE HYDROCHLORIDEDORZOLAMIDE HYDROCHLORIDE 279D961EN300-19768601DORZOLAMIDE HYDROCHLORIDE COMPONENT OF COSOPTL-671152SR-05000001449-3Q27106441Dorzolamide hydrochloride, European Pharmacopoeia (EP) Reference StandardDorzolamide for system suitability, European Pharmacopoeia (EP) Reference StandardDorzolamide hydrochloride, United States Pharmacopeia (USP) Reference Standard(2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H-1lambda6-thienothiopyran-6-sulfonamide hydrochloride(4S,6S)-4-(ethylamino)-5,6-dihydro-6-methyl-, 7,7-dioxide 4H-Thienothiopyran-2-sulfonamide hydrochloride122028-16-44H-Thieno(2,3-b)thiopyran-2-sulfonamide, 5,6-dihydro-4-(ethylamino)-6-methyl-, 7,7-dioxide, monohydrochloride, (4S,6S)-Molecular FormulaC10H17ClN2O4S3Molecular Weight360.901InChIInChI=1S/C10H16N2O4S3.ClH/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16;/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16);1H/t6-,8-;/m0./s1InChI KeyOSRUSFPMRGDLAG-QMGYSKNISA-NCanonical SMILES
Patent InformationPatent IDTitlePublication DateUS2015/191485Process for Preparing Enantiomerically Enriched Oxamides2015WO2008/75155CARBONIC ANHYDRASE INHIBITORS DERIVATIVES2008US2006/142595Process for preparing 5,6-dihydro-4-(S)-(ethylamino)-6-(S) methyl-4H-thienothiopyran-2-sulphonamide-7,7-dioxide HCI2006US2006/155132PYRROLOTRIAZINE DERIVATIVES2006
Physical Data
AppearanceA white to off white c1ystalline powder.
Melting Point, °C Solvent (Melting Point) 283 - 285238H2O
Density, g·cm-3Reference Temperature, °CMeasurement Temperature, °C1.60619.85
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts1Hdimethylsulfoxide-d6400Chemical shifts1Hdimethylsulfoxide-d6400Chemical shifts1Hdimethylsulfoxide-d6Chemical shifts13Cdimethylsulfoxide-d6
3-Aminopyridine CAS#: 462-08-8 NMR
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Temperature (IR Spectroscopy), °CFT-IRBandsKBr14.85 - 54.85
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)Comment (UV/VIS Spectroscopy)Absorption Maxima (UV/VIS), nmExt./Abs. Coefficient, l·mol-1cm-1Absorption maximaaq. HCl, methanol254
Route of Synthesis (ROS)
Route of Dorzolamide Hydrochloride CAS#:130693-82-2
ConditionsYieldStage #1: 5,6-dihydro-(S)-4-(ethylamino)-(S)-6-methyl-4H-thienothiopyran-2-sulfonamide 7,7-dioxide maleate salt With hydrogenchloride; sodium hydroxide In water at 52℃; pH=7.7; Inert atmosphere;Stage #2: With hydrogenchloride In water; isopropyl alcohol at 20 - 75℃;99.2%Stage #1: 5,6-dihydro-(S)-4-(ethylamino)-(S)-6-methyl-4H-thienothiopyran-2-sulfonamide 7,7-dioxide maleate salt With sodium hydroxide In water at 52℃; Inert atmosphere;Stage #2: With hydrogenchloride In water; isopropyl alcohol at 75℃;8.1 gExperimental Procedure 10 (4S,6S)-4-(ethylamino)-5,6-dihydro-6-methyl-4H-thienothiopyran-2-sulfonamide-7,7-dioxide, monochloride Dorzolamide HydrochlorideExample 10(4S,6S)-4-(ethylamino)-5,6-dihydro-6-methyl-4H-thienothiopyran-2-sulfonamide-7,7-dioxide, monochloride Dorzolamide HydrochlorideSodium hydroxide (8.0 g, 30% aqueous solution) was added to a suspension of trans-(6S)-4-ethylamino-5,6-dihydro-6-methyl-7,7-dioxo-4H-thienothiopyran-2-sulfonamide maleate salt (12.0 g, 27.2 mmol) in water (35 mL) at 52° C. under nitrogen atmosphere.The pH was adjusted to 7.7 with hydrochloric acid (2.3 g, 31.5% aqueous solution) and the mixture was diluted with ethyl acetate (36.1 g).The phases were separated and the aqueous phase counter-extracted with ethyl acetate (24.8 g).The phases were separated and the combined organic phases were washed with water (10.2 g) and concentrated up to a volume of approximately 30 mL.The mixture was then diluted with isopropyl alcohol (26.5 g) and the distillation process continued.The residue was diluted with isopropyl alcohol (33.0 g), and hydrochloric acid (4.6 g, 31.5% aqueous solution) was added to the mixture at 75° C.The temperature was brought to 20° C., the solid was filtered and washed with isopropyl alcohol (14.6 g, in 2 portions), then dried to give (4S,6S)-4-(ethylamino)-5,6-dihydro-6-methyl-4H-thienothiopyran-2-sulfonamide-7,7-dioxide, monochloride (8.1 g, titre 99.2%, ee 100%).1H NMR: δH (ppm) (400 MHz, DMSO) 9.9 (bs, 1H, -NH2+Cl-), 9.6 (bs, 1H, -NH2+Cl-), 8.2 (s, 2H, SO2NH2), 8.0 (s, 1H, CH), 4.7 (bs, 1H, CH), 4.35 (m, 1H, CH), 3.2 (bs, 1H, NH-CH2), 3.0 (bs, 1H, NH-CH2), 2.8 (m, 1H, CH2), 2.6-2.5 (m, 1H, CH2), 1.4 (d, 3H, J=6 Hz, CH3), 1.3 (t, 3H, J=7 Hz, CH3).
Safety and Hazards
Pictogram(s)SignalWarningGHS Hazard StatementsH302 (100%): Harmful if swallowed H373 (89.69%): Causes damage to organs through prolonged or repeated exposure Precautionary Statement CodesP260, P264, P270, P301+P317, P319, P330, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationUnder the room temperature and away from lightHS CodeStorageUnder the room temperature and away from lightShelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight360.907logP-0.23HBA6HBD2Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)151.33Rotatable Bond (RotB)3Matching Veber Rules1
BioactivityIn vitro: EfficacyQuantitative Results
Quantitative Results1 of 31Comment (Pharmacological Data)Bioactivities presentReferenceOphthalmic composition containing a carbonic anhydrase inhibitor and xanthan gum2 of 10 Comment (Pharmacological Data)Bioactivities presentReferenceCarbonic anhydrase inhibitors: Water-soluble 4-sulfamoylphenylthioureas as topical intraocular pressure-lowering agents with long-lasting effects3 of 31Comment (Pharmacological Data)Bioactivities presentReferenceEffects of some ophthalmic medications on pupil size: A literature review4 of 31Comment (Pharmacological Data)Bioactivities presentReferencePROCESS FOR PREPARING ENANTIOMERICALLY ENRICHED OXAMIDES5 of 31Comment (Pharmacological Data)Bioactivities presentReferenceCost-utility of primary open-angle glaucoma in Brazil6 of 31Comment (Pharmacological Data)Bioactivities presentReferenceBenzenesulfonamides Incorporating Flexible Triazole Moieties Are Highly Effective Carbonic Anhydrase Inhibitors: Synthesis and Kinetic, Crystallographic, Computational, and Intraocular Pressure Lowering Investigations
Toxicity/Safety PharmacologyQuantitative Results
Use PatternDorzolamide Hydrochloride CAS#:130693-82-2 is a carbonic anhydrase inhibitor. Dorzolamide hydrochloride is an anti-glaucoma topical eye drops. Dorzolamide hydrochloride is used to reduce the increased intraocular pressure in open angle glaucoma and ocular hypertension
Read the full article
0 notes