#Infectious Testing Market News
Text
Test Makers Target Monkeypox Market as Cases Surge | World News
Test Makers Target Monkeypox Market as Cases Surge | World News
LONDON (Reuters) – Diagnostic companies are racing to develop tests for monkeypox, hoping to tap into a new market as governments ramp up efforts to trace the world’s first major outbreak of the viral infection outside of Africa.
The scramble started last month, much like early 2020 when companies rushed to make kits to help diagnose COVID-19, creating a multibillion-dollar boon for test…

View On WordPress
#cases#Collections: World#Coronavirus#epidemics#Europe#infectious diseases#Makers#market#Monkeypox#Netherlands#News#public health#Reuters#Surge#Switzerland#target#Test#United States#Vaccines#World#World News
0 notes
Text
The Weather
A study in Clinical Infectious Diseases reported “that the risk of developing symptomatic illness within 14 days was 5 times greater when contacts were exposed to an asymptomatic [COVID]-positive child in their household.” Nearly 11% of household contacts developed symptoms within 14 days of exposure. The study also found, during a 3-month follow-up, that 6 out of 77 asymptomatic children developed Long COVID. The likelihood of developing symptoms from asymptomatic exposure is higher than we might expect. Continue to spread awareness of asymptomatic spread and advocate for increased infection control measures at your local schools.
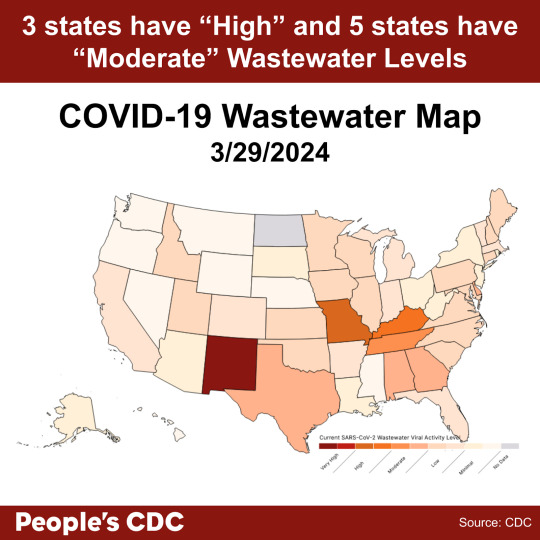
COVID wastewater levels are decreasing. As of 3/29/24, New Mexico is “Very High,” Arkansas and Kentucky are “High,” and the rest of the states are “Moderate” to “Low” levels of SARS-CoV-2 detected in wastewater.
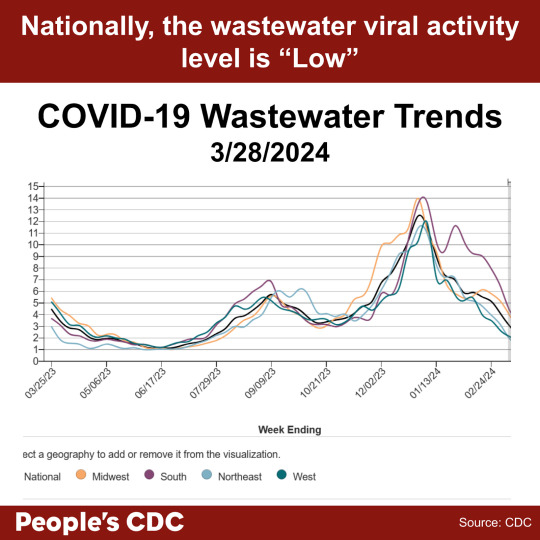
Wastewater levels continue to show a downward trend in the provisional data (gray shaded area) in all regions. The national wastewater levels are overall indicated as “Low.” While lower wastewater levels indicate decreased spread, it is important to continue to take precautions against infection. Holidays and spring breaks may bring people in closer proximity, so be sure to wear a mask to protect yourself and your community.
Wins
As we work to take more actions against the removal of vital public health measures, we remind you that you can still watch the recording of the People’s CDC press conference from March 13 and read the press release here. We would also like to remind you of the pre-proof of the People’s CDC External Review in the American Journal of Preventive Medicine Focus. The publication highlights the shortcomings of the CDC’s approach to public health and recommends a more equitable pandemic response.
News sources have published articles about the frustrations of people who continue to take COVID precautions. Time Magazine published an article presenting “both sides,” highlighting protest from people working with the CDC and concern from citizens and experts alike. While we are glad to see our voices be published in popular media, we are also saddened that “returning to normal” under economic and political pressure is so valued.
Treatments
Invyvid has received an FDA emergency use authorization for Pemgarda, a pre-exposure prophylaxis (PrEP) for people with immunocompromising conditions. Pemgarda is approved for people 12 and older with moderate to severe immunocompromise who are less likely to produce an adequate immune response to COVID vaccination alone. According to a press release from Invyvid, Pemgarda will release to market “imminently.”
Pre-exposure prophylaxis is commonly used for folks at high risk for exposure to HIV. As access to PrEP for HIV has been instrumental in keeping people safe, we hope that PrEP for COVID will be a useful tool for our community members with immune compromise. We also urge you to continue to wear high-quality masks and take other precautions to protect those most vulnerable.
Long COVID
People Magazine recently published an article highlighting an essay by Ziyad Al-Aly, physician and clinical epidemiologist, that pools data from several studies showing that COVID infection has lasting impacts on brain health. The review points out several impacts to cognitive functioning, including memory loss, spatial reasoning, and planning. Additionally, imaging studies have shown significant impact to brain tissue from inflammation, among other processes. The publication may be validating to those who experience lower cognitive function following COVID infection, including brain fog and memory dysfunction.
Take Action
We know that taking precautions–including masking, testing, and improving air quality–helps prevent the spread of airborne viral infection. Introducing more stringent precautions slowed outbreaks in the hematology ward of a hospital. The CDC recently released tips to improve ventilation. Help us urge the CDC to take other measures, including reinstating isolation periods.
Additionally, the home Test to Treat program is ending in April 2024. The program provides un-or-underinsured adults with free COVID and flu tests. If a participant in the program tests positive, they can also receive free healthcare via telehealth services. Join us to help save the program that helps so many at-risk people!
#op#covid#covid19#covid-19#covid 19#pcdc#people's cdc#long covid#covid pandemic#covid news#covid conscious#covid isn't over#pandemic#sars cov 2#coronavirus pandemic#coronavirus#sars-cov-2#cdc#prep#hiv#covid treatment#prep for covid#prep for covid-19#immunocompromised#disability#medical#uspol#img#links#described in alt text
2 notes
·
View notes
Text
Last week, in his State of the Union address, President Joe Biden told the American public that “we have broken COVID’s grip on us.” Highlighting the declines in the rates of COVID deaths, the millions of lives saved, and the importance of remembering the more than 1 million lost, Biden reminded the nation of what was to come: “Soon we’ll end the public-health emergency.”
When the U.S.’s state of emergency was declared nearly three years ago, as hospitals were overrun and morgues overflowed, the focus was on severe, short-term disease. Perhaps in that sense, the emergency is close to being over, Deeks told me. But long COVID, though slower to command attention, has since become its own emergency, never formally declared; for the millions of Americans who have been affected by the condition, their relationship with the virus does not yet seem to be in a better place.
Even with many more health-care providers clued into long COVID’s ills, the waiting lists for rehabilitation and treatment remain untenable, Hannah Davis told me. “I consider myself someone who gets exceptional care compared to other people,” she said. “And still, I hear from my doctor every nine or 10 months.” Calling a wrap on COVID’s “emergency” phase could worsen that already skewed supply-demand ratio. Changes to the nation’s funding tactics could strip resources—among them, access to telehealth; Medicaid coverage; and affordable antivirals, tests, and vaccines—from vulnerable populations, including people of color, that aren’t getting their needs met even as things stand, McCorkell told me. And as clinicians internalize the message that the coronavirus has largely been addressed, attention to its chronic impacts may dwindle. At least one of the country's long-COVID clinics has, in recent months, announced plans to close, and Davis worries that more could follow soon.
Scientists researching long COVID are also expecting new challenges. Reduced access to testing will complicate efforts to figure out how many people are developing the condition, and who’s most at risk. Should researchers turn their scientific focus away from studying causes and cures for long COVID when the emergency declaration lifts, Davids and others worry that there will be ripple effects on the scientific community’s interest in other, neglected chronic illnesses, such as ME/CFS (myalgic encephalomyelitis or chronic fatigue syndrome), a diagnosis that many long-haulers have also received.
The end of the U.S.’s official crisis mode on COVID could stymie research in other ways as well. At Johns Hopkins University, the infectious-disease epidemiologists Priya Duggal, Shruti Mehta, and Bryan Lau have been running a large study to better understand the conditions and circumstances that lead to long COVID, and how symptoms evolve over time. In the past two years, they have gathered online survey data from thousands of people who both have and haven’t been infected, and who have and haven’t seen their symptoms rapidly resolve. But as of late, they’ve been struggling to recruit enough people who caught the virus and didn’t feel their symptoms linger. “I think that the people who are suffering from long COVID will always do their best to participate,” Duggal told me. That may not be the case for individuals whose experiences with the virus were brief. A lot of them “are completely over it,” Duggal said. “Their life has moved on.”
Kate Porter, a Massachusetts-based marketing director, told me that she worries about her family’s future, should long COVID fade from the national discourse. She and her teenage daughter both caught the virus in the spring of 2020, and went on to develop chronic symptoms; their experience with the disease isn’t yet over. “Just because the emergency declaration is expiring, that doesn’t mean that suddenly people are magically going to get better and this issue is going to go away,” Porter told me. After months of relative improvement, her daughter is now fighting prolonged bouts of fatigue that are affecting her school life—and Porter isn’t sure how receptive people will be to her explanations, should their illnesses persist for years to come. “Two years from now, how am I going to explain, ‘Well, this is from COVID, five years ago’?” she said.
A condition that was once mired in skepticism, scorn, and gaslighting, long COVID now has recognition—but empathy for long-haulers could yet experience a backslide. Nisreen Alwan, a public-health researcher at the University of Southampton, in the U.K., and her colleagues have found that many long-haulers still worry about disclosing their condition, fearing that it could jeopardize their employment, social interactions, and more. Long COVID could soon be slated to become just one of many neglected chronic diseases, poorly understood and rarely discussed.
— Long COVID is the emergency that won’t end
#katherine j. wu#long covid is the emergency that won't end#current events#science#medicine#biology#human biology#disability#chronic illness#ableism#covid 19#pandemic#long covid
9 notes
·
View notes
Text
Lyndata a Creative Web Development Company...
●
PATHNET HEALTHCARE
NOW LIVE...
www.pathnethealthcare.com
AN INFORMATIVE WEBSITE BY LYNDATA.COM
●
"We offer Diagnostic services by performing tests on State-of -Art equipment in shortest possible Turn-around-time with cutting edge technology and easy affordability we are committed to set new standards in Preventive medicine, women’s healthcare, infectious diseases, genetics and cancer, we are advancing the course of modern medicine. In our pursuit for excellence in Diagnostic, Our well-trained technical staff strives to continuously upgrade its knowledge base & engage in accepting and implementing of new techniques on regular basis.".
●
Who are we ?
We Are Lyndata a Professional Team with more than 7 Years experienced in Web Development and Digital Marketing operating, from Lucknow , #India.
●
What are we famous for?
We provide Web Development and Digital Marketing Services with utmost customer support.
●
VISIT OUR SITE👇
www.lyndata.com
#CallUs 👇
955 949 1123

#branding#ecommerce#ecommerce web development#ecommerceportal#grapicdesign#logo design#lyndataindia#paymentintegration#trending#web development company#digital marketing#website development#tumblrpost#artists on tumblr
3 notes
·
View notes
Text
Informative Report on Clinical Biomarker Market | BIS Research

Clinical Biomarkers are measurable indicators of biological processes, conditions, or states within an organism. These can include molecules, genes, proteins, cells, or other substances that are detected and measured to assess normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention
The global clinical biomarkers market was valued at $24.80 billion in 2023 and is expected to reach $53.20 billion by 2033, growing at a CAGR of 7.93% between 2023 and 2033.
Clinical Biomarker Overview
Clinical Biomarkers are measurable indicators, such as molecules, genes, or proteins, that signal biological processes, disease states, or responses to treatment within an organism.
The era of Clinical Biomarker market heralds a paradigm shift in healthcare, wherein treatments are tailored to individual patients based on their unique genetic makeup, biomarker profiles, and clinical
characteristics.
Role of Clinical Biomarkers
Prognosis and Disease Progression
In addition to diagnosis, biomarkers play a pivotal role in assessing disease severity, progression, and prognosis. By monitoring changes in biomarker levels over time, healthcare providers can tailor treatment plans and predict patient outcomes with greater accuracy.
Therapeutic Monitoring:
Biomarkers also serve as markers of treatment efficacy and safety. In fields such as oncology, for instance, tumor biomarkers can gauge the response to chemotherapy or targeted therapies, guiding adjustments to treatment regimens as needed
Download the report and get to know the interesting facts Click Here !
Market Dynamics
Market Drivers
Growing Demand for Clinical Biomarker Products
Increase in Industrial Activity in Clinical Biomarker Landscape
Environment Changes Provoking Swift Care and Diagnosis
Market Restraints
High Price of Products/Services Limiting Adoption of Clinical Biomarkers in Low-Income Countries
Complex Regulatory Frameworks Delaying Approval of New Clinical Biomarkers Tests
Discovering New Biomarkers Presents Difficulty
Market Opportunities
Technological Advancement in Biomarker Testing
Increased Research Funding for Executing Research and Development Exercise
Discovery of Novel Biomarkers Expanding Precision Medicine Horizons
Grab a look at our sample page click here!
Market Segmentation
By Product Type
By Clinical Area
By Technology
By End Users
Click here to visit our Precision medicine page !
China has been able to procure its place as one of the leading contributors to the clinical diagnostics market in the past five years. Major growth was significantly attributed to the increasing adoption of clinical biomarkers in oncology or rare disease space.
Uses of Clinical Biomarkers
Disease Diagnosis and Prognosis
Drug Development and Clinical Trials
Personalized Medicine
Prognosis Assessment
Cancer Detection and Monitoring
Cardiovascular Risk Assessment:
Infectious Diseases
Environmental and Occupational Exposure Monitoring
Key Players in Clinical Biomarker Market
Abbott Laboratories
Agilent Technologies, Inc.
ALCEN
Recent Developments in the Global Clinical Biomarkers Market
•In August 2023, Quest Diagnostics launched the AD-Detect test for Alzheimer’s disease in the U.S., offering consumers the first opportunity to acquire and evaluate a blood-based biomarker test for assessing the potential risks of developing AD
•In September 2023, Becton, Dickinson and Company partnered with Navigate BioPharma Services, Inc. to develop and commercialize flow cytometry-based companion diagnostics and clinical decision tools. The collaboration combined Navigate BioPharma's expertise in biomarker assay design for clinical trials with BD's extensive portfolio of flow cytometry instruments, reagents, software, and in vitro diagnostics (IVD) development services.
Key Question Answers
QWhat are the major market drivers, challenges, and opportunities in the global clinical biomarkers market?
Q What are the business development strategies, such as business expansion, acquisitions, and funding, which are implemented by the major players to sustain in the competitive market?
Q Which is the dominant product and service type developed by the leading and emerging players for clinical biomarkers?
QHow is each segment of the market expected to grow during the forecast period from 2023 to 2033?
Conclusion
Biomarkers play a pivotal role in disease diagnosis, prognosis assessment, drug development, clinical trials, and personalized treatment strategies. Their utility extends to diverse areas, including oncology, cardiology, neurology, infectious diseases, and environmental health.
The clinical biomarkers market is witnessing significant growth and innovation, driven by the increasing demand for personalized medicine, advancements in diagnostic technologies, and the expanding scope of applications across healthcare and research domains
0 notes
Text
Laboratory Microplates, Global Market Size Forecast, Top 10 Players Rank and Market Share
Laboratory Microplates Market Summary
According to the new market research report “Global Laboratory Microplates Market Report 2023-2029”, published by QYResearch, the global Laboratory Microplates market size is projected to reach USD 139.4 million by 2029, at a CAGR of 5.9% during the forecast period.

Figure. Global Laboratory Microplates Market Size (US$ Million), 2018-2029

Based on or includes research from QYResearch: Global Laboratory Microplates Market Report 2023-2029.
Market Drivers:
Growth in Life Sciences and Medical Diagnostics: The rapidly advancing biotechnology and medical fields require high-throughput experiments and efficient sample processing, which has contributed to the growing demand for Laboratory Microplatess.
Drug Development and Screening: The pharmaceutical industry requires large-scale drug screening and pharmacodynamic studies, and Laboratory Microplatess provide an efficient method to test multiple compounds or samples simultaneously.
Vaccines and clinical trials: Vaccine development and clinical trials require testing of large numbers of samples, and Laboratory Microplatess help scale up sample testing.
Laboratory Automation: Laboratory Microplatess can be integrated with automated laboratory equipment to increase experimental efficiency and precision and reduce human error.
Rapid diagnosis and biomarker research: Laboratory Microplatess can be used to detect biomarkers, which is helpful in cancer diagnosis, infectious disease detection and other fields.
Restraint:
Cost: High-quality Laboratory Microplatess are expensive to manufacture, which may limit adoption by some laboratories and research institutions.
Standardization issues: The lack of unified Laboratory Microplates standards may lead to incompatible products from different suppliers.
Complex sample handling: Complex sample preparation and handling can lead to experimental instability and reproducibility issues.
Opportunity:
Personalized Medicine: With the rise of personalized medicine, Laboratory Microplatess can be used to analyze samples from individual patients to guide the development of personalized treatment plans.
Nanotechnology and microfluidic technology: Laboratory Microplatess combine nanotechnology and microfluidic technology to achieve higher sensitivity, smaller sample volumes, and faster experimental speeds, expanding application fields.
Green Technologies: The development of alternatives to organic solvents and the rise of green laboratories may impact Laboratory Microplates materials and manufacturing processes to reduce environmental impact.
Data Analytics and Artificial Intelligence: The development of data analytics and artificial intelligence can improve the interpretation and utilization of results from Laboratory Microplates experiments, accelerating the discovery and application process.
Figure. Global Laboratory Microplates Top 10 Players Ranking and Market Share(Continually updated)
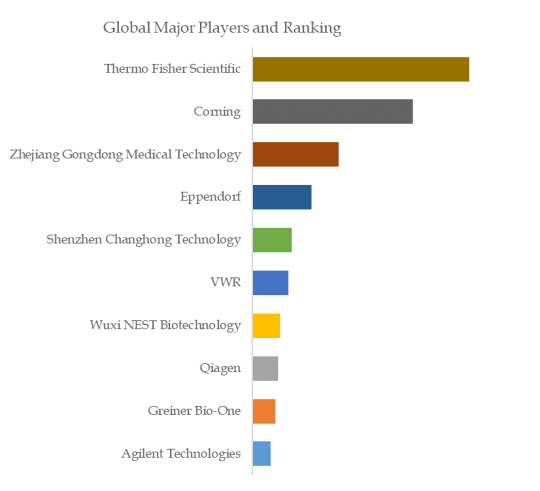
Based on or includes research from QYResearch: Global Laboratory Microplates Market Report 2023-2029.
Globally, major Laboratory Microplates manufacturers include Agilent Technologies, Greiner Bio-One, Qiagen, etc., among which the top five manufacturers account for approximately xx market share.
Figure. Laboratory Microplates, Global Market Size, Split by Product Segment

Based on or includes research from QYResearch: Global Laboratory Microplates Market Report 2023-2029.
In terms of product type, 96-Wellis the largest segment.
Figure. Laboratory Microplates, Global Market Size, Split by Application Segment

Based on or includes research from QYResearch: Global Laboratory Microplates Market Report 2023-2029.
In terms of product application, Pharmaceutical Companies is the largest application.
Figure. Laboratory Microplates, Global Market Size, Split by Region (Production)


Based on or includes research from QYResearch: Global Laboratory Microplates Market Report 2023-2029.
Figure. Laboratory Microplates, Global Market Size, Split by Region

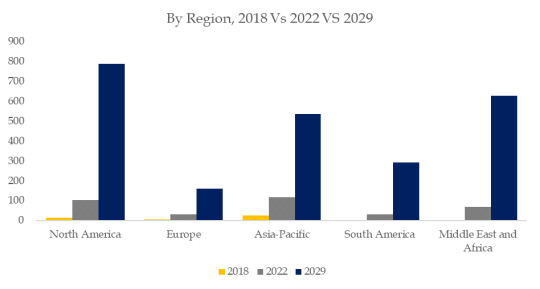
Based on or includes research from QYResearch: Global Laboratory Microplates Market Report 2023-2029.
About QYResearch
QYResearch founded in California, USA in 2007.It is a leading global market research and consulting company. With over 16 years’ experience and professional research team in various cities over the world QY Research focuses on management consulting, database and seminar services, IPO consulting, industry chain research and customized research to help our clients in providing non-linear revenue model and make them successful. We are globally recognized for our expansive portfolio of services, good corporate citizenship, and our strong commitment to sustainability. Up to now, we have cooperated with more than 60,000 clients across five continents. Let’s work closely with you and build a bold and better future.
QYResearch is a world-renowned large-scale consulting company. The industry covers various high-tech industry chain market segments, spanning the semiconductor industry chain (semiconductor equipment and parts, semiconductor materials, ICs, Foundry, packaging and testing, discrete devices, sensors, optoelectronic devices), photovoltaic industry chain (equipment, cells, modules, auxiliary material brackets, inverters, power station terminals), new energy automobile industry chain (batteries and materials, auto parts, batteries, motors, electronic control, automotive semiconductors, etc.), communication industry chain (communication system equipment, terminal equipment, electronic components, RF front-end, optical modules, 4G/5G/6G, broadband, IoT, digital economy, AI), advanced materials industry Chain (metal materials, polymer materials, ceramic materials, nano materials, etc.), machinery manufacturing industry chain (CNC machine tools, construction machinery, electrical machinery, 3C automation, industrial robots, lasers, industrial control, drones), food, beverages and pharmaceuticals, medical equipment, agriculture, etc.
0 notes
Text
Medical Swabs Market Future Scope Demands and Projected Industry Growths to 2031

The global medical swabs market has been experiencing significant growth over the past few years and is projected to continue its upward trajectory in the coming decade. According to recent market research, the market size was valued at USD 2.95 billion in 2022 and is expected to reach USD 5.01 billion by 2030, with a compound annual growth rate (CAGR) of 6.8% during the forecast period of 2023-2030. This remarkable growth can be attributed to various factors driving demand, along with emerging trends and opportunities reshaping the landscape of the medical swabs industry.
Emerging Trends and Opportunities:
Rising Demand for Point-of-Care Testing (POCT): With the increasing emphasis on early disease detection and rapid diagnosis, there has been a growing demand for point-of-care testing. Medical swabs play a crucial role in sample collection for various POCT applications, including infectious disease screening, genetic testing, and drug testing.
Advancements in Material and Design: Manufacturers are focusing on developing innovative swab designs and materials to enhance sample collection efficiency and patient comfort. The emergence of materials like flocked nylon and rayon, along with advanced designs such as foam-tipped swabs, is driving market growth.
Expanding Applications in Forensic and Veterinary Sciences: Medical swabs are not only used in clinical settings but also find applications in forensic investigations and veterinary diagnostics. The increasing adoption of DNA collection swabs in forensic analysis and sample collection in veterinary medicine is opening up new avenues for market players.
Download Free Sample Report: https://www.snsinsider.com/sample-request/2979
Key Drivers Propelling Growth:
Growing Emphasis on Infection Control: With the rise in healthcare-associated infections (HAIs) and the ongoing COVID-19 pandemic, there has been a heightened focus on infection control measures. Medical swabs are essential for specimen collection, environmental surface sampling, and wound care, driving their demand in healthcare facilities worldwide.
Increasing Number of Surgical Procedures: The rising prevalence of chronic diseases, coupled with an aging population, is leading to a surge in surgical procedures globally. Medical swabs are extensively used in surgical settings for preoperative skin preparation, wound cleaning, and specimen collection, fueling market growth.
Technological Advancements in Healthcare Infrastructure: The integration of advanced technologies such as robotics, automation, and artificial intelligence (AI) in healthcare infrastructure is streamlining sample collection processes and improving diagnostic accuracy. This technological evolution is boosting the demand for specialized medical swabs tailored for automated systems.
Challenges and Considerations:
Supply Chain Disruptions: The medical swabs market is susceptible to supply chain disruptions, especially during global health crises or natural disasters. Ensuring a resilient and diversified supply chain is crucial for mitigating risks and maintaining uninterrupted product availability.
Stringent Regulatory Compliance: Compliance with stringent regulatory requirements, including quality standards and product certifications, poses a challenge for market players. Adhering to regulatory frameworks while innovating and introducing new products requires substantial investments in research and development.
Price Sensitivity in Developing Regions: In developing regions, price sensitivity among healthcare providers and budget constraints in public healthcare systems may hinder market growth. Manufacturers need to adopt pricing strategies that accommodate diverse market segments without compromising on product quality.
Key Takeaways from the Market:
The medical swabs market is poised for substantial growth, driven by factors such as the increasing prevalence of infectious diseases, technological advancements, and the expanding applications beyond clinical settings.
Market players should focus on product innovation, strategic collaborations, and geographic expansion to capitalize on emerging opportunities and gain a competitive edge.
Addressing challenges related to supply chain resilience, regulatory compliance, and pricing strategies will be imperative for sustained growth and market penetration, particularly in diverse global markets.
In conclusion, the medical swabs market presents lucrative opportunities for manufacturers and stakeholders, fueled by evolving healthcare needs and technological advancements. By navigating the emerging trends, addressing key drivers, and overcoming challenges, players can unlock the full potential of this dynamic market and contribute to improving healthcare outcomes worldwide.
0 notes
Text
We are the Trusted Choice in Medical Grade Cleaning Products
When it comes to healthcare, hygiene is not only preferred, but it is a must. The need for sanitary workplaces is critical, and using the best cleaning supplies is necessary to meet this requirement. Proudly made in the USA, Medical Grade Cleaning Products have become the first line of defence against pollutants and infections in hospitals and other healthcare settings.
The cornerstone of Medical Grade Cleaning Products lies in their unparalleled quality. Manufactured in state-of-the-art facilities across the United States, these products undergo rigorous testing and adhere to the strictest standards set forth by regulatory bodies. From Disinfecting wipes to Disinfecting sprays and Hand sanitizers, each formulation is meticulously crafted to deliver optimal results in healthcare settings.
Hospitals, where the stakes are highest, rely on the efficacy of these cleaning solutions to maintain a safe and sanitary environment for patients, staff, and visitors alike. With the constant influx of individuals carrying various pathogens, the need for robust cleaning protocols is non-negotiable. Medical Grade Cleaning Products like Sanitising wipes provide hospitals with the confidence they need to combat infectious agents effectively, mitigating the risk of healthcare-associated infections.
The efficacy of Medical Grade Cleaning Products extends beyond the confines of healthcare facilities. From schools and offices to residential homes, these products have garnered widespread acclaim for their ability to deliver consistent results. Families across the nation trust in the power of these cleaners to safeguard their loved ones against harmful germs and bacteria, providing peace of mind in an uncertain world.
In addition to their efficacy, Medical Grade Cleaning Products prioritize safety. Formulated with environmentally friendly ingredients, these cleaners offer a powerful yet gentle solution for disinfection. Unlike harsh chemicals that can pose risks to both users and the environment, these products strike the perfect balance between effectiveness and safety, ensuring that cleaning tasks can be performed without compromise.
The "Made in the USA" label carries with it a sense of pride and assurance. With Medical Grade Cleaning Products, this sentiment rings especially true. By choosing domestically produced cleaners, consumers not only support local economies but also invest in products that adhere to stringent quality control measures. The decision to manufacture these cleaning solutions within the USA underscores a commitment to excellence and accountability.
Beyond their primary function of disinfection, Medical Grade Cleaning Products embody a commitment to sustainability. By opting for eco-friendly formulations, these cleaners minimize their impact on the environment without sacrificing performance. This eco-conscious approach resonates with consumers who prioritize sustainability in their purchasing decisions, further solidifying the appeal of these products in the market.
The landscape of cleaning products is ever-evolving, driven by advancements in technology and science. Medical Grade Cleaning Products remain at the forefront of this innovation, constantly pushing the boundaries of what is possible in terms of efficacy and safety. Whether it's the development of new formulations or the integration of cutting-edge delivery systems, these products continue to raise the bar for cleanliness standards across industries.
The trust placed in Medical Grade Cleaning Products is not unfounded. Backed by scientific research and real-world testing, these cleaners have earned their reputation as reliable solutions for disinfection. Consumers can rest assured knowing that when they choose these products, they are choosing excellence – a sentiment echoed by hospitals, businesses, and households nationwide.
The widespread adoption of Medical Grade Cleaning Products serves as a testament to their success. From their humble beginnings to their current status as industry leaders, these cleaners have stood the test of time, proving their worth in the most demanding of environments. As the healthcare landscape continues to evolve, one thing remains constant: the indispensable role of Medical Grade Cleaning Products in safeguarding public health.
In an age where cleanliness is paramount, Medical Grade Cleaning Products stand as beacons of excellence. Trusted by hospitals and loved by everyone, these domestically produced cleaners embody a commitment to quality, safety, and innovation. Made in the USA, they serve as a testament to the nation's ability to lead the way in the fight against pathogens and contaminants. As we navigate the challenges of tomorrow, one thing is certain: Medical Grade Cleaning Products will continue to be indispensable allies in our quest for cleanliness and safety.
0 notes
Text
Transformative Technologies: In Vitro Diagnostics in Focus
IVD refer to medical devices and tests that are used to analyze samples taken from the human body, such as blood, urine, and tissue. These samples are collected from patients and tested outside of a living body in controlled laboratory conditions. IVD assists in disease screening, diagnosis of infections like HIV, monitoring disease progression or regression, and making decisions regarding drug treatments and medical interventions.
Growing Demand and Market Size
The global IVD market was valued at $70 billion in 2020 and is projected to reach $126 billion by 2028, expanding at a CAGR of 7.3% during the forecast period. The rising burden of chronic and infectious diseases, technological advancements in miniaturization and automation, point-of-care testing, and personalized medicine are some of the key factors driving the growth of the IVD industry. Precision medicine and companion diagnostics are also creating new opportunities for IVD manufacturers to cater to unmet medical needs.
Emerging Technologies
Some of the emerging technologies revolutionizing the In Vitro Diagnostics landscape include:
Next-Generation Sequencing (NGS)
NGS allows the sequencing of millions of DNA fragments simultaneously at high speed and low cost. It is being widely used for genetic disease screening, cancer diagnosis through tumor mutational burden testing, infectious disease detection, pharmacogenomics, and non-invasive prenatal testing. Continuous advancements in NGS workflow automation, data analysis, and interpretation are making it more accessible for clinical use.
Lab-on-a-Chip Technology
Also known as microfluidics, lab-on-a-chip miniaturizes traditional benchtop laboratory tests onto a silicon chip a few square centimeters in size. It allows automation and parallel processing of multiple diagnostic assays with minimal sample volume requirements. Applications include point-of-care testing for infectious diseases and glucose monitoring. Further advancement can make lab-on-chip diagnostics affordable for use in resource-limited settings.
Digital and Molecular Diagnostics
The digitization of diagnostic processes allows automation and streamlining of pre-analytical, analytical, and post-analytical stages. Digital PCR, isothermal amplification techniques, and microarray-based molecular diagnostics offer high sensitivity and specificity for infectious disease detection, genetic disorders screening, and cancer monitoring. Integration of AI and machine learning is augmenting data analysis capabilities.
Advancement in Biosensors
Continued research into nanotechnology, materials science, and sensor fabrication is revolutionizing the development of biosensors for IVD applications. Electrochemical, optical, and mass-sensitive biosensors enable rapid, multiplexed, affordable, and on-site testing with high precision. Applications include glucose monitoring, genetic disease screening, cardiac marker testing, infectious agent detection for epidemics and bioterrorism threats.
Challenges and Standardization Needs
While emerging technologies hold immense potential to transform diagnostics, their clinical validation and regulatory approval remain long drawn processes. Achieving standardization in pre-analytical variables, performance metrics, quality control protocols, and data interpretation across decentralized locations poses difficulties. High initial investment and operational costs can delay the real-world adoption of advanced IVD technologies, especially in low to middle-income countries. Lack of skilled labor and infrastructure in resource-limited regions further hampers access to quality diagnostic services. Overcoming these challenges through partnerships, standardized guidelines, innovative business models, and human capital investments would be crucial to realize the full benefits of emerging IVD technologies.
Regulatory Changes and Global Harmonization
In vitro diagnostic regulators worldwide are aligning processes and requirements to facilitate the global development and distribution of new IVD technologies. The U.S. FDA is shifting from a risk-based to a total-product lifecycle approach through the implementation of the Verification and Validation framework. The European IVD Regulation establishes a single regulatory structure across EU markets. Global harmonization initiatives led by bodies like the World Health Organization aim to establish consistent standards and mutual recognition of approvals. Such regulatory changes intend to expedite patients' access to advanced diagnostics while maintaining pre-market evaluation of safety, efficacy, and performance.
Future Trends and Conclusion
The future of IVD looks promising with advancements spanning multiple omics technologies, digital platforms, lab miniaturization, and big data analytics. Integration of diagnostics into therapeutic strategies will become more prevalent. Radical new technologies like mobile health diagnostics, wearable biosensors, and molecular pathology could transform healthcare delivery models. Nonetheless, building robust research infrastructure, streamlining regulatory pathways, ensuring affordability, and addressing ethical issues would be pre-requisites to realize the full potential. IVD's crucial role in public health interventions and precision medicine will continue propelling innovations aimed at making diagnostics more accessible, non-invasive, rapid, accurate, and cost-effective.
0 notes
Text
Liquid Biopsy Market Latest Innovations, Drivers and Industry Status 2023 to 2030
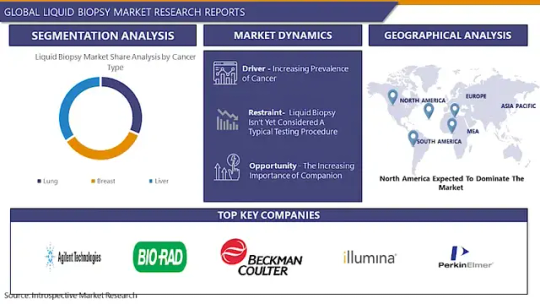
The Global Liquid Biopsy Market size is expected to grow from USD 1.59 billion in 2022 to USD 7.02 billion by 2030, at a CAGR of 20.4% during the forecast period (2023-2030).
A liquid biopsy is a minimally invasive diagnostic procedure that involves analyzing various biomarkers (such as circulating tumor cells, cell-free DNA, exosomes, and other nucleic acids) found in bodily fluids like blood, urine, or saliva. It's primarily used for the detection and monitoring of diseases, particularly cancer.
Advances in technologies such as next-generation sequencing (NGS), polymerase chain reaction (PCR), and digital PCR have greatly enhanced the sensitivity and specificity of liquid biopsy tests, enabling more accurate detection and monitoring of diseases.
Liquid biopsies have gained particular attention in the field of oncology for early cancer detection, monitoring treatment response, and detecting resistance mutations. They offer a non-invasive alternative to tissue biopsies and can provide real-time information on tumor dynamics.
While oncology remains the primary focus of liquid biopsy applications, researchers and companies have been exploring its potential in other areas such as prenatal testing, infectious disease diagnostics, transplant monitoring, and autoimmune disease detection.
Get Full PDF Sample Copy of Report: (Including Full TOC, List of Tables & Figures, Chart) @
https://introspectivemarketresearch.com/request/16300
Updated Version 2024 is available our Sample Report May Includes the:
Scope For 2024
Brief Introduction to the research report.
Table of Contents (Scope covered as a part of the study)
Top players in the market
Research framework (structure of the report)
Research methodology adopted by Worldwide Market Reports
Leading players involved in the Liquid Biopsy Market include:
Agilent Technologies (United States), Bio-Rad Laboratories, Inc. (United States), Beckman Coulter(United States), Illumina (United States), PerkinElmer (United States), Becton, Dickinson and Company (BD) (United States), Johnson & Johnson (United States), Abbott Laboratories (United States), Cancer Genetics, Inc. (United States), Myriad Genetics, Inc. (United States)
Moreover, the report includes significant chapters such as Patent Analysis, Regulatory Framework, Technology Roadmap, BCG Matrix, Heat Map Analysis, Price Trend Analysis, and Investment Analysis which help to understand the market direction and movement in the current and upcoming years.
If You Have Any Query Liquid Biopsy Market Report, Visit:
https://introspectivemarketresearch.com/inquiry/16300
Segmentation of Liquid Biopsy Market:
By Cancer Type
Lung
Breast
Liver
By Circulating Biomarker
Circulating Tumor DNA
Circulating Tumor Cells
By End-User
Hospitals
Laboratories
Government Research Centre
By Regions: -
North America (US, Canada, Mexico)
Eastern Europe (Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe)
Western Europe (Germany, UK, France, Netherlands, Italy, Russia, Spain, Rest of Western Europe)
Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New Zealand, Rest of APAC)
Middle East & Africa (Turkey, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa)
South America (Brazil, Argentina, Rest of SA)
Key Reasons To Invest In Liquid Biopsy Market Report:
To provide a complete structure and a basic overview of the Liquid Biopsy market.
To provide insights into important Liquid Biopsy aspects such as growth trajectory, CAGR value, market share, and revenue analysis.
Assess growth opportunities, threats, market drivers, and associated risks.
To understand the Liquid Biopsy market competition by analysing the top business people along with market profiles, import/export details, revenue, profit, and market shares.
Indicate pricing structure, import/export details, supply chain analysis, SWOT analysis to facilitate key decision-making process.
Analysing emerging Liquid Biopsy market segments and sub-segments to drive ultimate growth, investment analysis, and future growth opportunities.
Understand sources of knowledge, intended research methodology, and important conclusions.
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
Acquire This Reports: -
https://introspectivemarketresearch.com/checkout/?user=1&_sid=16300
About us:
Introspective Market Research (introspectivemarketresearch.com) is a visionary research consulting firm dedicated to assisting our clients to grow and have a successful impact on the market. Our team at IMR is ready to assist our clients to flourish their business by offering strategies to gain success and monopoly in their respective fields. We are a global market research company, that specializes in using big data and advanced analytics to show the bigger picture of the market trends. We help our clients to think differently and build better tomorrow for all of us. We are a technology-driven research company, we analyse extremely large sets of data to discover deeper insights and provide conclusive consulting. We not only provide intelligence solutions, but we help our clients in how they can achieve their goals.
Contact us:
Introspective Market Research
3001 S King Drive,
Chicago, Illinois
60616 USA
Ph no: +1-773-382-1047
Email: [email protected]
#Liquid Biopsy#Liquid Biopsy Market#Liquid Biopsy Market Size#Liquid Biopsy Market Share#Liquid Biopsy Market Growth#Liquid Biopsy Market Trend#Liquid Biopsy Market segment#Liquid Biopsy Market Opportunity#Liquid Biopsy Market Analysis 2023
0 notes
Text
Antimicrobial Susceptibility Market: Precision in Pathogen Control
Antimicrobial Susceptibility Testing Market
The worldwide antimicrobial susceptibility testing market is expected to increase at a 5.5% CAGR from 2022 to 2027, from an anticipated value of US$3.6 billion in 2022 to US$4.7 billion in 2027. The Antimicrobial Susceptibility Testing (AST) Technology Market is expanding due to factors such as rising infectious illness prevalence, increased antibiotic resistance, and favourable government measures to minimise infectious disease burden. Antibiotic susceptibility testing is becoming more popular due to the rising prevalence of certain illnesses, such as bloodstream infections and pneumonia caused by E. coli, K. pneumonia, and P. aeruginosa, among others.
Click here for full report:
https://www.pharmanucleus.com/reports/antimicrobial-susceptibility-market
Antimicrobial Susceptibility Testing Market Dynamics
Drivers: Emergence of multidrug resistance due to drug abuse
The rise of drug resistance poses a significant threat to global health, particularly in the context of antibiotics. Antibiotics are becoming less effective as drug-resistant strains of bacteria spread worldwide. This has profound implications for disease treatment, as it makes infections more difficult to treat and ultimately increases mortality rates.
The World Health Organization (WHO) has identified drug-resistant pathogens, such as carbapenem-resistant gram-negative bacteria, as a major concern. Urgent development of new drugs is necessary to combat these infections effectively. However, without a fundamental change in the way antibiotics are used, the effectiveness of these new treatments may be compromised.
Misuse and overuse of antibiotics contribute to the development of drug resistance. Factors such as inappropriate prescribing, improper self-medication, and the use of antibiotics in livestock and agriculture all contribute to the problem. These practices create an environment where bacteria can adapt and develop resistance mechanisms, rendering antibiotics less effective over time.
To preserve the efficacy of antibiotics, it is crucial to promote responsible antibiotic use. This includes raising awareness among healthcare providers and the general public about appropriate antibiotic prescribing and the importance of completing prescribed courses of treatment. Additionally, robust regulations and surveillance systems are needed to monitor antibiotic use in various sectors, including healthcare, agriculture, and veterinary medicine.
Efforts to develop new antibiotics should be complemented by strategies to optimize their use, such as antibiotic stewardship programs and infection prevention and control measures. Research into alternative treatment options, such as phage therapy and immunotherapies, is also essential to diversify the arsenal against drug-resistant bacteria.
Opportunities: Growth opportunities in emerging market
The antimicrobial susceptibility testing market players are poised with significant opportunities in developing countries such as APAC (Asia-Pacific), India, China, and Japan. These countries are experiencing a notable growth in the antimicrobial susceptibility testing market, driven by several factors.
Firstly, the booming medical tourism industry in these regions attracts a large influx of patients seeking healthcare services, including diagnostics. This surge in medical tourism creates a demand for antimicrobial susceptibility testing as part of the diagnostic process.
Secondly, the adult population (over 20 years old) is growing in these countries, leading to a higher prevalence of infectious diseases. As a result, there is an increased need for accurate and timely antimicrobial susceptibility testing to guide appropriate treatment decisions.
Additionally, the increasing use of drugs in these regions contributes to the demand for antimicrobial susceptibility testing. With a growing population and expanding healthcare access, the requirement for testing to guide optimal drug selection and treatment outcomes becomes crucial.
Moreover, these countries possess a strong and skilled workforce that is well-versed in high technology. This skilled workforce plays a vital role in the successful implementation and operation of advanced antimicrobial susceptibility testing technologies, ensuring accurate and reliable results.
Furthermore, the relatively lax import/export laws in these regions provide an advantageous environment for market players to expand their brands and offerings. The underdeveloped nature of certain areas in these countries presents opportunities for companies to introduce and establish their technologies, filling gaps in the market and addressing unmet needs.
The growing number of technicians and their desire to learn and adopt new technologies further contribute to the development and updating of antimicrobial susceptibility testing capabilities in these regions, fostering innovation and advancements in the field.
Click here for full report:
https://www.pharmanucleus.com/reports/antimicrobial-susceptibility-market
Challenges: Complex Regulatory Frameworks
One of the major challenges in antibiotic susceptibility testing is the time gap between establishing clinical thresholds, obtaining regulatory approval for innovative antimicrobial treatments, and bringing those drugs to market. The process of gaining approval for new drugs involves stringent guidelines set by regulatory bodies like the FDA, which can result in significant delays.
The lack of established clinical thresholds for certain drugs further complicates the use of products aimed at increasing the susceptibility of microorganisms. Clinical thresholds are important benchmarks used to determine the effectiveness and appropriate usage of antimicrobial therapies. In some cases, such as invasive mold infections other than Aspergillus, the efficacy of antifungal therapy cannot be accurately assessed without the availability of specific clinical thresholds.
This time lag and absence of clinical thresholds can hinder the timely utilization of innovative antimicrobial treatments. It delays access to potentially life-saving therapies and limits healthcare professionals' ability to make informed decisions regarding treatment choices. The gap between setting clinical thresholds, regulatory approval, and market availability needs to be addressed to ensure that effective antimicrobial treatments reach patients in a timely manner and contribute to the fight against drug-resistant microorganisms.
Click here for request free sample:
https://www.pharmanucleus.com/request-sample/1193
Restraints: High Cost of automated Laboratory Instruments
Automated equipment for antimicrobial laboratories is quite expensive and offers premium features and functionality. The introduction of automated AST systems shortens incubation and detection times. Another barrier to this market is the high maintenance cost of these devices, which come with the latest software and require regular maintenance.
#antimicrobial susceptibility#pathogen control#infection management#precision medicine#microbial resistance solutions
0 notes
Text
Revolutionizing Healthcare: The Promise of Precision Medicine

In the ever-evolving landscape of healthcare, one concept is rapidly gaining traction for its potential to transform treatment outcomes and patient care: precision medicine. This innovative approach represents a paradigm shift, moving away from the traditional one-size-fits-all approach to healthcare and towards personalized, tailored treatments that are uniquely suited to each individual's genetic makeup. At the heart of precision medicine lies the groundbreaking field of genomics, which enables the comprehensive analysis of an individual's genetic information. Let's explore how precision medicine is reshaping the future of healthcare and the pivotal role of pharmaceutical contract manufacturing in bringing these revolutionary therapies to patients.
Unraveling the Mysteries of Precision Medicine
Precision medicine is founded on the principle that each person's genetic makeup influences their susceptibility to diseases, their response to treatments, and their overall health outcomes. By analyzing an individual's genome, scientists can identify genetic variations that may predispose them to certain diseases or influence their response to specific medications. Armed with this knowledge, healthcare providers can tailor treatments to target the underlying molecular mechanisms of diseases, maximizing efficacy and minimizing side effects.
The Power of Genomics in Healthcare
Advancements in genomics have been instrumental in unlocking the potential of precision medicine. The ability to sequence an individual's entire genome with unprecedented speed and accuracy has paved the way for a deeper understanding of the genetic basis of diseases. From cancer to cardiovascular disorders, genomics research has shed light on the complex interplay between genetics, environment, and lifestyle factors in disease development. This deeper understanding has led to the identification of novel drug targets, biomarkers for early disease detection, and predictive tools for treatment response.
Precision Medicine in Action: Real-World Applications
The impact of precision medicine is already being felt across a wide range of medical specialties. In oncology, for example, molecular profiling of tumors allows clinicians to match patients with targeted therapies that address specific genetic mutations driving cancer growth. Similarly, in cardiovascular medicine, genetic testing can identify individuals at increased risk of inherited heart conditions, enabling early interventions to prevent adverse events. Beyond cancer and cardiovascular diseases, precision medicine holds promise for a multitude of conditions, including neurological disorders, rare genetic diseases, and infectious diseases.
The Role of Pharmaceutical Contract Manufacturing in Precision Medicine
As the field of precision medicine continues to expand, the pharmaceutical industry plays a pivotal role in translating scientific discoveries into life-changing therapies. Pharmaceutical contract manufacturing, with its expertise in formulation development, scale-up, and production, is instrumental in bringing precision medicines from the lab to the clinic. Contract manufacturing organizations (CMOs) work closely with pharmaceutical companies to manufacture specialized drugs, biologics, and gene therapies tailored to individual patients' needs.
Embracing the Future of Healthcare: A Call to Action
As we stand on the cusp of a new era in healthcare, it is essential to recognize the transformative potential of precision medicine. By harnessing the power of genomics and personalized treatments, we can revolutionize the way we prevent, diagnose, and treat diseases. However, realizing this vision requires collaboration, innovation, and investment across the healthcare ecosystem.
Pharmaceutical contract manufacturing plays a crucial role in this endeavor, providing the expertise and infrastructure needed to bring precision medicines to market efficiently and cost-effectively. As such, I urge pharmaceutical companies, healthcare providers, policymakers, and patients alike to embrace the promise of precision medicine and support initiatives that advance its development and accessibility.
Together, let us harness the power of precision medicine to usher in a new era of healthcare, where treatments are as unique as the individuals they serve. By leveraging the expertise of pharmaceutical contract manufacturers, we can accelerate the pace of innovation and bring hope to patients in need.
Conclusion
Precision medicine represents a seismic shift in our approach to healthcare, offering the potential to revolutionize treatment paradigms and improve patient outcomes. With advances in genomics driving the development of personalized therapies, the future of medicine has never looked brighter. As we embark on this transformative journey, let us embrace the opportunities presented by precision medicine and collaborate to make its benefits accessible to all. Together, we can shape a future where healthcare is truly personalized, effective, and equitable.
0 notes
Text

Global Nucleic Acid Isolation and Purification Market size at USD 4.22 billion in 2023. During the forecast period between 2024 and 2030, BlueWeave expects the Global Nucleic Acid Isolation and Purification Market size to expand at a CAGR of 9.23% reaching a value of USD 8.47 billion by 2030. Growing R&D efforts and an increase in the use of sequencing platforms for clinical diagnostics are two key growth drivers for the Global Nucleic Acid Isolation and Purification Market. The is also expected to be driven by an increase in genomics research as well as new product launches.
Opportunity: Rising application for diagnostics
The Global Nucleic Acid Isolation and Purification Market is segmented into drug discovery & development, precision medicine, diagnostics, agriculture and animal research, and other, based on application. The diagnostics segment holds the largest share in the Global Nucleic Acid Isolation and Purification Market. According to research published on ScienceDirect, RNA/DNA genetic tests are the most reliable for identifying infectious illnesses and determining a person's vulnerability to certain diseases, including COVID-19. Drug discovery and development is anticipated to register the highest growth rate during the forecast period.
Impact of Escalating Geopolitical Tensions on Global Nucleic Acid Isolation and Purification Market
The escalating geopolitical tensions restrict the growth of the Global Nucleic Acid Isolation and Purification Market. For instance, Ukraine has been emerging as the leading destination for clinical research and trials of various diseases, particularly cancer. The presence of an organized and digitized health system makes it cheaper to accommodate medical research in Ukraine compared to many other Western European nations. However, Russia’s invasion of Ukraine disrupted the medical research ecosystem in the country. Many reports reveal that Ukraine has a large number of clinical research specialists as well as infrastructure, which directly impacts the demand for nucleic acid isolation and purification, hindering its global market growth.
Sample Request @ https://www.blueweaveconsulting.com/report/nucleic-acid-isolation-and-purification-market/report-sample
0 notes