#estimated glomerular filtration rate
Text
Jazmin Evans had been waiting for a new kidney for four years when her hospital revealed shocking news: She should have been put on the transplant list in 2015 instead of 2019 — and a racially biased organ test was to blame.
As upsetting as that notification was, it also was part of an unprecedented move to mitigate the racial inequity. Evans is among more than 14,000 Black kidney transplant candidates so far given credit for lost waiting time, moving them up the priority list for their transplant.
“I remember just reading that letter over and over again,” said Evans, 29, of Philadelphia, who shared the notice in a TikTok video to educate other patients. “How could this happen?”
At issue is a once widely used test that overestimated how well Black people’s kidneys were functioning, making them look healthier than they really were — all because of an automated formula that calculated results for Black and non-Black patients differently. That race-based equation could delay diagnosis of organ failure and evaluation for a transplant, exacerbating other disparities that already make Black patients more at risk of needing a new kidney but less likely to get one.
A few years ago, the National Kidney Foundation and American Society of Nephrology prodded laboratories to switch to race-free equations in calculating kidney function. Then the U.S. organ transplant network ordered hospitals to use only race-neutral test results in adding new patients to the kidney waiting list.
“The immediate question came up: What about the people on the list right now? You can’t just leave them behind,” said Dr. Martha Pavlakis of Boston’s Beth Israel Deaconess Medical Center and former chair of the network’s kidney committee.
Pavlakis calls what happened next an attempt at restorative justice: The transplant network gave hospitals a year to uncover which Black kidney candidates could have qualified for a new kidney sooner if not for the race-based test — and adjust their waiting time to make up for it. That lookback continues for each newly listed Black patient to see if they, too, should have been referred sooner.
Between January 2023 and mid-March, more than 14,300 Black kidney transplant candidates have had their wait times modified, by an average of two years, according to the United Network for Organ Sharing, which runs the transplant system. So far more than 2,800 of them, including Evans, have received a transplant.
But it’s just one example of a larger problem permeating health care. Numerous formulas or “algorithms” used in medical decisions — treatment guidelines, diagnostic tests, risk calculators — adjust the answers according to race or ethnicity in a way that puts people of color at disadvantage.
Given how embedded these equations are in medical software and electronic records, even doctors may not realize how widely they impact care decisions.
“Health equity scholars have been raising alarm bells about the way race has been misused in clinical algorithms for decades,” said Dr. Michelle Morse, New York City’s chief medical officer.
Change is beginning, slowly. No longer are obstetricians supposed to include race in determining the risk of a pregnant woman attempting vaginal birth after a prior C-section. The American Heart Association just removed race from a commonly used calculator of people’s heart disease risk. The American Thoracic Society has urged replacing race-based lung function evaluation.
The kidney saga is unique because of the effort to remedy a past wrong.
“Lots of time when we see health inequities, we just assume there’s nothing we can do about it,” Morse said. “We can make changes to restore faith in the health system and to actually address the unfair and avoidable outcomes that Black people and other people of color face.”
Black Americans are over three times more likely than white people to experience kidney failure. Of the roughly 89,000 people currently on the waiting list for a new kidney, about 30% are Black.
Race isn’t a biological factor like age, sex or weight — it’s a social construct. So how did it make its way into calculations of kidney function?
The eGFR, or estimated glomerular filtration rate, evaluates kidney health based on how quickly a waste compound called creatinine gets filtered from blood. In 1999, an equation used to calculate eGFR was modified to adjust Black people’s results compared to everyone else’s, based on some studies with small numbers of Black patients and a long-ago false theory about differences in creatinine levels. Until recently that meant many lab reports would list two results — one calculated for non-Black patients and another for Black patients that could overestimate kidney function by as much as 16%.
Not every Black kidney candidate was affected. Some may have had kidney failure diagnosed without that test. For others to have a chance at benefitting from UNOS’ mandated lookback, transplant center staff-turned-detectives often worked after hours and weekends, hunting years-old records for a test that, recalculated without the race adjustment, might make the difference.
“You’re reaching out to the nephrologist, their primary care doctors, the dialysis units to get those records,” said Dr. Pooja Singh of Jefferson Health’s transplant institute in Philadelphia, where Evans received her new kidney. “That first patient getting transplanted for us was such a great moment for our program that the work didn’t feel like work after that.”
A high school sports physical first spotted Evans’ kidney disease at age 17. While finishing her master’s degree and beginning to earn her Ph.D. at Temple University, she started dialysis — for nine hours a night while she slept — and was placed on the transplant list.
How long it takes to get a kidney transplant depends on patients’ blood type, medical urgency and a mix of other factors — including how long they’ve spent on the waiting list. Evans was first listed in April 2019. When the Jefferson transplant center unearthed her old lab tests, they found she should have qualified in September 2015.
“Just for context, when I was still an undergrad I should have been on the list,” she said, recalling the anger she felt as she read the letter. What she called “a mind-blowing” credit of 3½ more years waiting also provided “a glimmer of hope” that she’d be offered a matching kidney soon.
Evans got a new kidney on July 4 and is healthy again, and grateful the policy change came in time for her.
“You don’t know if people would be alive today” if it had been enacted earlier, she said. Still, that extra step of “making amends to fix the situation for those that we can — I feel like it’s very important and it’s very necessary if you’re truly wanting to bring more equity and equality into the medical field.”
#us politics#news#ap#2024#the associated press#kidney transplant#racial bias#us healthcare#eGFR#estimated glomerular filtration rate#transplant surgery
4 notes
·
View notes
Text
Maintained Renal Function by Blood Pressure Control in Patient with Diabetic Kidney Disease (DKD) | Abstract
View On WordPress
#Anti-Hypertensive Agents#Chronic Kidney Disease Epidemiology Collaboration#Estimated Glomerular Filtration Rate#Low Carbohydrate Diet#Type-2 Diabetes
0 notes
Text
Understanding Kidney Function Test
A kidney function test is a blood or urine test that checks how well your kidneys are working. The kidneys remove waste products and excess fluid from the body.
#blood urea nitrogen (BUN) level#Creatinine level#Estimated Glomerular Filtration Rate (eGFR) Urinalysis
0 notes
Text
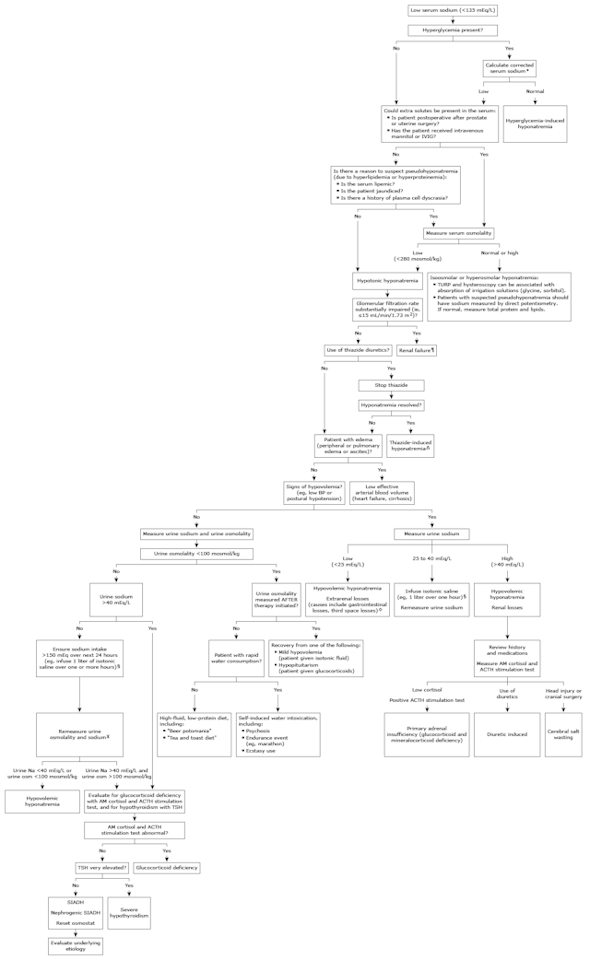
Hyponatremia, defined as a serum sodium concentration below 135 mEq/L, is usually caused by a failure to excrete water normally. In healthy individuals, the ingestion of water does not lead to hyponatremia because suppressed release of antidiuretic hormone (ADH), also called vasopressin, allows excess water to be excreted in a dilute urine.
●The initial diagnostic approach to the adult patient with hyponatremia consists of a directed history and physical examination as well as selected laboratory tests. When hyponatremia is first discovered, some elements of the history, key features of the physical exam, and the results of several helpful laboratory tests are usually already available, and these guide the subsequent diagnostic approach:
•If hyperglycemia is present, the serum sodium concentration should be corrected for the effect of glucose to exclude hypertonic hyponatremia. To calculate the "corrected" serum sodium, we recommend the use of the following ratio: the sodium concentration will fall by approximately 2 mEq/L for each 100 mg/100 mL (5.5 mmol/L) increase in glucose concentration.
•Patients with lipemic serum, severe obstructive jaundice, or a known plasma cell dyscrasia may have pseudohyponatremia. This laboratory artifact can occur if the sodium is measured with flame photometry or indirect potentiometry using ion-selective electrodes when the solid phase portion of serum or plasma is increased due to severe elevations of triglycerides, lipoprotein-X, or protein. The true concentration of sodium in plasma water can be measured using direct ion-selective electrodes, which are not susceptible to the artifact. Such direct ion-selective electrodes are utilized by most "point of care" bedside analyzers and devices used to measure blood gases. In addition, patients with pseudohyponatremia typically have a normal serum osmolality.
•Patients who have had recent surgery utilizing large volumes of electrolyte-poor irrigation fluid (eg, prostate or intrauterine procedures) and those treated with mannitol, glycerol, or intravenous immune globulin may have isotonic or hypertonic hyponatremia. Measurement of the plasma osmolality is helpful in these settings.
•Patients who do not have hyperglycemia or one of these other features associated with pseudohyponatremia, isotonic hyponatremia, or hypertonic hyponatremia are likely to have hypotonic hyponatremia.
●The serum creatinine concentration, which can be used to estimate glomerular filtration rate (GFR), and the patient's medication history are typically available at the time that hyponatremia is discovered. Both severely reduced GFR and thiazide (or thiazide-type) diuretics are important causes of hypotonic hyponatremia.
●In patients with hypotonic hyponatremia who do not have severely reduced GFR and who are not taking a thiazide diuretic, or in patients suspected of having an additional cause of hyponatremia, the subsequent evaluation depends upon whether or not the patient has clinically apparent edema and/or ascites:
•Patients with hyponatremia due to heart failure or cirrhosis typically have advanced disease and present with clinically apparent peripheral edema and/or ascites along with a previous diagnosis of heart or liver failure.
•Nonedematous patients with hypotonic hyponatremia are either euvolemic or hypovolemic. Most patients with hyponatremia due to true hypovolemia will have obvious signs of volume depletion; however, some hypovolemic patients have more subtle signs and are mistakenly judged to be euvolemic. The evaluation of nonedematous patients usually requires further testing:
-Hyponatremic patients who present with clinical symptoms and signs of hypovolemia may have extrarenal fluid losses or renal fluid losses. Measurement of the urine sodium and chloride concentrations can often distinguish between these two causes.
-Most hyponatremic patients who appear to be euvolemic by physical examination have the syndrome of inappropriate ADH (SIADH). However, such patients may occasionally have hyponatremia due to true volume depletion, primary polydipsia, malnutrition, glucocorticoid deficiency, or severe hypothyroidism. The subsequent evaluation in such patients includes measurement of the urine sodium and urine osmolality as well as levels of cortisol and thyroid-stimulating hormone.
6 notes
·
View notes
Text
Understanding African American and non-African American eGFR laboratory results
Understanding why eGFR laboratory reports include African American and non-African American results
Glomerular filtration rate (GFR) is the best way to measure how well your kidneys are working, but this test is complicated and cannot be easily done in a doctor’s office. To get around this, laboratories use mathematical equations to estimate the glomerular filtration rate instead of measuring it. This is why laboratories report estimated GFR or eGFR.
Two commonly used estimating equations for eGFR are the CKD MDRD (Modification of Diet in Renal Disease) and the CKD EPI (Chronic Kidney Disease Epidemiology Collaboration) equations. Using these math equations, eGFR is calculated from the amount of creatinine in the blood.
Creatinine is a waste product that comes from the normal wear and tear on your body’s muscles and also from the foods you eat. Everyone has creatinine in their bloodstream. However, creatinine levels can differ between people. This reason for this difference may not only be related to kidney disease – it may be affected by several other factors, such as age, sex, and body weight.
Race was originally included in eGFR calculations because clinical trials demonstrated that people who self-identify as Black/African American can have, on average, higher levels of creatinine in their blood. It was thought the reason why was due to differences in muscle mass, diet, and the way the kidneys eliminate creatinine. Since a patient’s race is not always used when laboratory tests are ordered, laboratories used different eGFR calculations for African American and non-African American and included both numbers in their lab results.
The use of race in calculating eGFR has been a subject of debate. Race is not a biological concept, but a social construct. Using race as a factor for calculating eGFR does not account for the diversity within communities of color. Also, people who self-identify as multiracial might not want to be put in a single racial bucket.
What is the NKF doing to address concerns about using race in measuring eGFR?
In 2020, the National Kidney Foundation (NKF) and the American Society of Nephrology (ASN) formed a joint task force to review the use of race in eGFR calculations. On September 23, 2021, the Task Force announced a new race-free calculation for estimating eGFR. The National Kidney Foundation is working with the nation’s laboratories to get this new calculation into use as quickly as possible. The NKF is also creating materials to show how this new equation may impact patient care.
How is eGFR used?
Estimated GFR is one of the key tests for diagnosing kidney disease. The earlier kidney disease is detected, the better the chance of managing it or keeping your condition from getting worse. Your eGFR informs healthcare professionals about your kidney function and helps them to recommend the best treatments for you. For instance, eGFR results are used in the following ways:
Confirming that kidney function is normal for a potential living kidney donor
Making sure the right dose of medicine is used
Enrollment in clinical trials that use kidney function as an inclusion or exclusion criterion
Making sure the right type of imaging tests and dyes are used
When to refer someone to a nephrologist or kidney doctor
If and when to plan for dialysis
When to start an evaluation for a kidney transplant
Although the NKF alone cannot solve all inequalities, the NKF is working to help identify, confront and reverse them. NKF advocates for making affordable healthcare more available and making sure that communities of color are not left behind. NKF’s CARES helpline is available to all people with kidney disease. NKF’s educational offerings include the impact of kidney disease on diverse communities, and the reasons why communities of color have been disproportionately affected by COVID-19. NKF is committed to continuing its work to address inequalities in kidney health.

#gfr#african american#kemetic dreams#african#africans#lab test#laboratory#health#clincial#clinical laboratory test market
7 notes
·
View notes
Text
Does Metformin, a glucose-lowering drug, hurt the kidneys?
Metformin is a biguanide compound that reduces blood sugar mainly by reducing hepatic glucose output, improving insulin resistance, and reducing glucose absorption in the small intestine. It is currently one of the world's most widely used oral hypoglycemic drugs. Drug safety evaluation studies have found that Metformin has a good safety profile, no carcinogenic or mutagenic effects, and no evidence that Metformin can increase the risk of lactic acidosis. Medicilon has a professional team and experience in preclinical drug safety evaluation services, providing high-quality data and a fast turnaround time to support all drug safety evaluation studies.
Many patients are concerned about the effects of long-term metformin use on the kidneys. The drug does not directly damage the kidneys but can lead to drug accumulation when taken by patients with existing kidney damage. Both the Chinese Guidelines for the Prevention and Treatment of Type 2 Diabetes (2017 edition) and the Expert Consensus on the Clinical Application of Metformin (2016 edition) suggest that Metformin is the drug of choice for the treatment of type 2 diabetes in the absence of contraindications and intolerance, and should always be retained in the glucose-lowering regimen.
1, Benefits of Metformin.
Metformin can reduce hyperglycemia with no hypoglycemic effect on those with regular blood sugar; the drug has the following benefits in addition to hypoglycemia.
(1), Metformin has the effect of reducing body weight.
(2) Metformin has apparent cardioprotective effects and reduces the risk of cardiovascular disease in newly diagnosed and established type 2, diabetes patients.
(3) Metformin can improve lipid synthesis, metabolism, and lipid profile.
(4) Metformin significantly improved liver serological enzyme profile and metabolic abnormalities in patients with non-alcoholic fatty liver.
2, Adverse reactions and countermeasures
The main adverse reactions of Metformin are diarrhea, nausea, vomiting, gastric distension, and other gastrointestinal responses, which mainly occur in the early stage of treatment (the majority happen in the first ten weeks). Most patients can gradually tolerate them, or their symptoms disappear as the duration of treatment increases. Start taking small doses, gradually increase the amount, adjust the quantity at the right time, take with meals, and choose enteric preparations and other methods, which can reduce gastrointestinal reactions.
Three 、Does Metformin hurt the liver and kidney?
Metformin has no hepatic and renal toxicity; Metformin is absorbed through the gastrointestinal tract for blood circulation, almost does not combine with plasma albumin, does not go through liver metabolism, does not compete with liver P450 enzymes, and does not degrade in the body, but acts directly on the liver and muscle, reducing hepatic glucose isomerism and increasing muscle glucose enzymes. Therefore, Metformin is not hepatotoxic.
Metformin is mainly excreted from the urine in its original form by the kidneys and is cleared rapidly, with approximately 90% clearance in 12-24h. The renal clearance of Metformin is about 3.5 times higher than that of creatinine, and renal tubular excretion is the main route of metformin clearance. Therefore, Metformin itself is not harmful to the kidney.
However, caution should be exercised when using Metformin in people with impaired liver and kidney function. Metformin should be avoided when serum transaminases exceed three times the upper limit of normal, and patients with renal insufficiency need to adjust the dose by estimating the level of glomerular filtration rate. Clinicians or pharmacists can assess the above.
4, long-term use of Metformin, the need for appropriate supplementation of vitamin B12
Studies have shown that: the incidence of vitamin B12 deficiency in glucose patients using Metformin is 5.8%, while the incidence of vitamin B12 deficiency in glucose patients not using Metformin and people without diabetes is 2.4% and 3.3%, respectively. Therefore, long-term metformin users should monitor vitamin B12 concentration regularly and increase the intake of vitamin B12-rich foods appropriately (vitamin B12 is mainly contained in animal proteins, such as meat, animal liver, fish, shellfish, eggs, etc.) to prevent and correct vitamin B12 deficiency. If this condition occurs, vitamin B12 should be supplemented in an appropriate amount under the guidance of professional doctors.
Five 、Stop taking Metformin 48 hours before and after doing a CT examination
Diabetic patients should stop taking Metformin 48 hours before and after doing enhanced CT because it is necessary to play contrast agent before doing CT. The contrast agent belongs to macromolecular substances excreted through the kidneys. If you retake Metformin, it will increase the burden on the kidney and cause contrast nephropathy.
6, the icing on the cake: combined with other glucose-lowering drugs
There are several different drugs when taking glucose-lowering drugs, often more than Metformin. This is because the combination of glucose-lowering medications mutually increases the hypoglycemic effect, improves insulin resistance, or reduces adverse reactions.
All diabetic patients should choose the appropriate hypoglycemic drugs according to their different conditions and pay attention to the indications and contraindications of each type of drug. At the same time, it is essential to emphasize that diet therapy and diet control are the cure for diabetic patients and must be adhered to for life. Diet therapy should not be relaxed or abandoned because of oral hypoglycemic drugs or increased dosages of hypoglycemic medications.
3 notes
·
View notes
Text
Painkillers Can Sometimes Increase Chronic Pain
This test measures the amount of a waste product in your blood that is normally removed by your kidneys. If your kidneys are not working as well as they should, the creatinine level will be increased in your blood. The results of the serum creatinine test can be used to estimate your glomerular filtration rate . Your GFR number tells your doctor how much kidney function you have. A unique kidney disease patient registry that will advance patient education and kidney disease treatment. Corticosteroids are often administered as an injection at the site of musculoskeletal injuries.
Many people have questions surrounding the difference between these two terms. As it turns out, both terms are often interchanged because these substances largely produce the same effects. Oxycodone is sold under brand names including OxyContin and Percocet.
It also includes access to her popular Helping Older Parents Course and live QA calls with her. Medrol is a powerful anti-inflammatory steroid, and a form of steroid can also be injected directly into the joint which is often the way it’s used in a rotator cuff tear. Some types of rotator cuff injury require a surgical repair unfortunately. I haven’t hears of that before but I think “panadol” is also known as paracetamol, which is the same as Tylenol , so you could consider it as being similar to the acetaminophen discussed in the article.
It should be made clear that absolutely no one decides to become addicted to prescription painkillers. No Arctic Blast Review and thinks about how they’re now going to alienate their loved ones, lose their job, or become involved on the wrong side of law enforcement. However, while the abuse of illegal street drugs is on the decline in the United States, the abuse of prescription painkillers in on the rise.
Unfortunately, painkillers aren’t without their fair share of risks. And when speaking specifically about powerful opioids like OxyContin or Percocet, painkillers can have very negative effects on your body. In this article, we’ll review how pain pills work and discuss the long-term effects painkillers can have on the body. Yet many current and former players interviewed by ESPN said it was commonplace for players to get prescription painkillers from sources outside of NFL locker rooms, either from unscrupulous doctors or drug dealers.
#back pain relief#pain relief#pain relief frequency#lower back pain relief#knee pain relief#joint pain relief#shoulder pain relief#sciatica pain relief#upper back pain relief#pain relief meditation#leg pain relief#gas pain relief#neck pain relief#muscle pain relief#chronic pain relief#natural pain relief
2 notes
·
View notes
Text
The eGFR, or estimated glomerular filtration rate, evaluates kidney health based on how quickly a waste compound called creatinine gets filtered from blood. In 1999, an equation used to calculate eGFR was modified to adjust Black people’s results compared to everyone else’s, based on some studies with small numbers of Black patients and a long-ago false theory about differences in creatinine levels. Until recently that meant many lab reports would list two results — one calculated for non-Black patients and another for Black patients that could overestimate kidney function by as much as 16%.
0 notes
Text
The Significance of Comprehensive Health Checkups in Peritoneal Dialysis:
Regular full-body health evaluations are essential for maximizing outcomes and improving long-term survival among individuals undergoing peritoneal dialysis (PD). These assessments encompass a range of diagnostic techniques aimed at evaluating various health aspects, including kidney function, cardiovascular health, nutritional status, and metabolic indicators. Key elements of these evaluations for PD patients include:
1. Kidney Function Assessment: Routine monitoring of parameters such as serum creatinine, blood urea nitrogen (BUN), and estimated glomerular filtration rate (eGFR) aids in the early detection of declining kidney function. This information guides decisions on dialysis adequacy and modality selection.
2. Cardiovascular Screening: Thorough cardiovascular evaluations, including electrocardiography (ECG), echocardiography, and cardiac biomarker tests, provide insights into cardiac health and function. Early identification and management of cardiovascular risk factors like hypertension, dyslipidemia, and coronary artery disease are crucial for preventing cardiovascular complications and improving survival rates among PD patients.
3. Nutritional Status Assessment: Monitoring nutritional status through biochemical markers (e.g., serum albumin, prealbumin, and total protein), anthropometric measurements, and dietary assessments helps in early detection of malnutrition. This information guides interventions aimed at optimizing protein-energy status and preserving lean body mass in PD patients.
4. Metabolic Evaluation: Assessing metabolic parameters such as serum electrolytes, glucose, lipid profile, and markers of mineral and bone metabolism (e.g., calcium, phosphorus, and parathyroid hormone) assists in detecting and managing metabolic imbalances common in PD patients. Optimizing these parameters is crucial for reducing the risk of metabolic bone disease, cardiovascular calcification, and other metabolic complications associated with end-stage renal disease (ESRD).
There are several good hospitals in India that offer health checkup packages to choose from based on an individual's health status and requirements. A regular full body health checkup helps in increasing the survival rate of patients undergoing peritoneal dialysis.
#dialysis#peritoneal dialysis#full body health checkup#regular health checkups#health checkup packages#kidney failure#kidney function test#creatinine levels#BUN#kidney function#ECG#echocardiogram#nutritional status#lipid profile#blood glucose
0 notes
Text
"Hyponatremia may be a marker of increased risk for dementia, investigators reported at the American Society of Nephrology’s Kidney Week 2022 conference in Orlando, Florida.
In a post hoc analysis of the Systolic Blood Pressure Intervention Trial SPRINT-MIND, 129 of 8541 (1.5%) patients with normal baseline serum sodium experienced hyponatremia at 6 months with a mean serum sodium of 127 meq/L and a total of 324 events.
Hyponatremia was significantly associated with a 2.5- and 2.1-fold increased risk for probable dementia over a mean 4.8 years in an unadjusted model and a fully adjusted model, respectively, Srinivasan Beddhu, MD, of the University of Utah Health in Salt Lake City, reported on behalf of his team. It was not associated with mild cognitive impairment, however. The investigators adjusted for the systolic blood pressure intervention, age, sex, race, cardiovascular disease, congestive heart failure, smoking, body mass index (BMI), and estimated glomerular filtration rate." -
https://www.renalandurologynews.com/reports/hyponatremia-linked-to-dementia/#:~:text=Hyponatremia%20was%20significantly%20associated%20with,on%20behalf%20of%20his%20team.
0 notes
Text
"Navigating Chronic Kidney Disease: Understanding, Management, and Support"
Chronic kidney disease (CKD) is a progressive condition characterized by the gradual loss of kidney function over time. It affects millions of people worldwide and poses significant challenges to both patients and healthcare systems. This article provides an in-depth exploration of CKD, covering its causes, symptoms, diagnosis, and management strategies.
Causes: CKD can result from various underlying conditions that impair kidney function. The most common causes include hypertension (high blood pressure) and diabetes mellitus. Other factors contributing to CKD include autoimmune diseases, polycystic kidney disease, recurrent kidney infections, urinary tract obstructions, and prolonged use of certain medications. Understanding the root cause of CKD is crucial for determining the appropriate treatment approach.
Symptoms: In its early stages, CKD may be asymptomatic, making it challenging to detect without medical screening. However, as the condition progresses, individuals may experience symptoms such as fatigue, weakness, swelling in the ankles, feet, or hands (edema), shortness of breath, difficulty concentrating, decreased appetite, and changes in urination patterns, including increased frequency or decreased urine output. Recognizing these symptoms is essential for timely diagnosis and intervention.
Diagnosis: Diagnosing CKD typically involves a combination of medical history assessment, physical examination, and laboratory tests. Blood tests measure creatinine levels and estimate glomerular filtration rate (eGFR), providing insights into kidney function. Urine tests can detect the presence of protein or blood in the urine, indicating kidney damage. Imaging studies such as ultrasound, CT scans, or MRI may be utilized to assess kidney structure and identify any abnormalities. Early detection through routine screenings is critical for slowing disease progression and preventing complications.
Management: Management of CKD aims to slow the progression of kidney damage, alleviate symptoms, and minimize complications. Lifestyle modifications play a central role and may include dietary changes to control blood pressure, blood sugar levels, and reduce the intake of sodium, phosphorus, and potassium. Regular exercise, smoking cessation, and maintaining a healthy weight are also recommended. Medications such as ACE inhibitors or angiotensin receptor blockers may be prescribed to manage hypertension and protect kidney function. In advanced stages of CKD, dialysis or kidney transplantation may be necessary to replace lost kidney function and improve quality of life.
Additionally, patient education and ongoing monitoring are essential components of CKD management. Patients should be informed about their condition, including potential complications, medication adherence, and lifestyle modifications. Regular follow-up appointments with healthcare providers allow for adjustments to treatment plans based on disease progression and individual needs.
In conclusion, chronic kidney disease is a significant health concern with far-reaching implications. By understanding its causes, recognizing symptoms, and implementing appropriate management strategies, individuals with CKD can optimize their health outcomes and quality of life. Early intervention and proactive management are key to mitigating the impact of this chronic condition.
0 notes
Text
Prediction for the Progression of Chronic Kidney Disease (CKD) in Various Situations | Abstract
Prediction for the Progression of Chronic Kidney Disease (CKD) in Various Situations | Abstract
View On WordPress
#Chronic Kidney Disease#End-Stage Kidney Disease#Estimated Glomerular Filtration Rate#Kidney Replacement Therapy#Urinary Alb/Cre Ratio
0 notes
Text
Understanding Kidney Function Tests: Importance and Accessibility in Nigeria
Kidney Function tests in Nigeria are vital diagnostic tools used to assess the health and functionality of the kidneys. In Nigeria, where kidney disease is a significant health concern, these tests play a crucial role in early detection, management, and treatment. Understanding the importance and accessibility of kidney function tests is paramount for promoting kidney health and reducing the burden of kidney-related illnesses in the country.

Chronic kidney disease (CKD) is a growing problem in Nigeria, affecting a significant portion of the population. The causes are diverse, including diabetes, hypertension, infections, and lifestyle factors. Early detection of kidney dysfunction is critical to prevent progression to end-stage renal disease (ESRD) and its associated complications. Kidney function tests serve as valuable tools in identifying abnormalities in kidney function at an early stage, allowing for timely intervention and management.
One of the primary tests used to evaluate kidney function is serum creatinine measurement. Creatinine is a waste product generated by muscle metabolism and is excreted by the kidneys. Elevated levels of serum creatinine indicate decreased kidney function, signaling potential kidney damage or dysfunction. Another crucial test is the estimation of glomerular filtration rate (eGFR), which provides an assessment of the kidneys' ability to filter waste products from the blood. These tests, along with others such as blood urea nitrogen (BUN) and urine albumin-to-creatinine ratio, form the cornerstone of kidney function evaluation.
Accessibility to kidney function tests in Nigeria, however, remains a challenge, particularly in rural areas and underserved communities. Limited healthcare infrastructure, insufficient diagnostic facilities, and high costs pose significant barriers to accessing these essential tests for many Nigerians. Additionally, lack of awareness and education about kidney health further exacerbates the problem, leading to delayed diagnosis and poor management of kidney-related conditions.
To address these challenges, concerted efforts are needed to improve the availability and affordability of kidney function tests across the country. This includes investing in healthcare infrastructure, expanding diagnostic facilities, and subsidizing the cost of tests to make them more accessible to the general population, especially those in remote areas. Furthermore, public health campaigns aimed at raising awareness about the importance of kidney health and the significance of regular screening can help encourage early detection and prevention of kidney disease.
In addition to enhancing access to Laboratory tests in Nigeria; there is a need to strengthen healthcare systems to ensure prompt diagnosis and effective management of kidney-related conditions. This involves training healthcare professionals in the interpretation of test results, implementing evidence-based guidelines for kidney disease management, and promoting multidisciplinary care approaches to address the complex needs of patients with kidney disease.
In conclusion, Diagnostics services in Nigeria is a crucial role in the early detection and management of kidney disease in Nigeria. Improving accessibility to these tests, along with raising awareness about kidney health, is essential for reducing the burden of kidney-related illnesses and improving the overall health outcomes of the population. By prioritizing kidney health and investing in preventive measures, Nigeria can take significant strides towards combating the growing challenge of kidney disease in the country.
#kidney function tests in nigeria#diagnostics services in nigeria#medical diagnostic centres in nigeria#laboratory tests in nigeria
0 notes
Text
Update
she doesn't know I'm under her bed but got her blood results back.
Lipid Profile:
Total Cholesterol: 180 mg/dl
LDL (Low-Density Lipoprotein): 110 mg/dl
HDL (High-Density Lipoprotein): 50 mg/dl
Triglycerides: 120 mg/dl
Liver Function Tests:
AST (Aspartate Aminotransferase): 25 U/L
ALT (Alanine Aminotransferase): 30 U/L
ALP (Alkaline Phosphatase): 70 U/L
Bilirubin: 0.8 mg/dl
Renal Function Tests:
Blood Urea Nitrogen (BUN): 10 mg/dl
Serum Creatinine: 0.8 mg/dl
Estimated Glomerular Filtration Rate (eGFR): 90 mL/min/1.73m²
so she should in theory be happy (I hope so)
0 notes
Text
I need to read about dosing and management of lithium. There is a lot of stuff you have to monitor for pts taking lithium and I don't feel comfortable prescribing it. But I don't have time to read this right now. Will look at this later and fix with page breaks: Laboratory tests and monitoring — Before prescribing lithium and during ongoing treatment, laboratory tests need to be obtained because lithium can adversely affect several organ systems. Management of abnormal test results and adverse effects resulting from lithium are described elsewhere in the topic, as is management of a positive pregnancy test. (See 'Renal' below and 'Thyroid' below and 'Parathyroid' below and 'Cardiac' below and 'Pregnancy' below.) Prior to beginning lithium, the following tests should be obtained [47-51]: ●Urinalysis, blood urea nitrogen, creatinine, thyroid function studies, and calcium. ●Pregnancy test for women of childbearing potential. ●Electrocardiogram for patients with risk factors for coronary heart disease, including diabetes mellitus, hypertension, dyslipidemia, and cigarette smoking. (See "Overview of established risk factors for cardiovascular disease".) Lithium levels should be checked five to seven days after the dose is changed. In addition, a lithium level should be checked if a dose increase is under consideration and a level has not been measured for at least two weeks. Patients on steady doses should have their levels checked every 6 to 12 months. In addition to checking lithium levels during ongoing treatment, renal, thyroid, and parathyroid function should be monitored as follows [10,44,47-51]: ●Urinalysis, blood urea nitrogen, and creatinine every two to three months during the first six months of therapy, and every 6 to 12 months thereafter. (See "Renal toxicity of lithium".) ●Thyroid function tests once or twice during the first six months, and every 6 to 12 months thereafter or more frequently in higher risk patients. (See "Lithium and the thyroid".) ●Serum calcium is monitored yearly. MANAGING LITHIUM ADVERSE EFFECTS Lithium can cause many acute and long-term adverse effects that are not necessarily associated with toxicity [52]. Some adverse effects, such as weight gain and cognitive impairment, are more likely to be associated with nonadherence than other adverse effects, such as nausea, polyuria/polydipsia, and tremor [44]. Severe or a sudden worsening of side effects may be a sign of lithium toxicity. (See 'Lithium toxicity' above and "Lithium poisoning".) General strategies — General strategies for managing adverse effects of lithium include [44]: ●Watchful waiting – Tolerance to some side effects (eg, nausea and tremor) can eventually occur, but is unlikely with other adverse effects (eg, weight gain). ●Changing the time of administration. ●Lowering the dose; however, dose reductions risk compromising efficacy. ●Changing to a different lithium formulation (immediate or slow release). ●Dividing the daily dose to take smaller amounts more often, to decrease peak serum levels. ●Treating adverse effects with a second drug (eg, diuretic for polyuria/polydipsia). ●Discontinuing lithium and switching to a different drug if adverse effects are intolerable and cannot be managed. Strategies for managing specific adverse effects are discussed in the sections below. Renal — Renal function is adversely affected by lithium and thus monitored with laboratory tests [50]. (See 'Laboratory tests and monitoring' above.) Adverse renal effects are often functional and reversible, but may eventually progress to structural, permanent changes [44,53,54]. As an example, a study examined the impact of long-term lithium treatment (mean duration 18 years) on glomerular filtration rate in 312 bipolar disorder patients (mean age = 56 years) [55]. Extended lithium exposure reduced the estimated glomerular filtration rate by approximately 30 percent more than that associated with aging alone. Additional risk factors for decreased estimated glomerular filtration rate were higher serum lithium concentrations, longer exposure, lower initial estimated glomerular filtration rate, general medical comorbidity, and older age. None of the patients developed end-stage kidney disease. Abnormal renal function tests are managed in collaboration with a nephrologist to determine the need for further testing, a reduction in the dose of lithium, or switching to an alternative medication for treating bipolar disorder. Additional information about lithium and renal toxicity is discussed separately. (See "Renal toxicity of lithium".) Polyuria and polydipsia — Polyuria is often defined as urinating more than three liters in 24 hours [44]. Polyuria and polydipsia have been observed in up to 70 percent of lithium treated patients; potential risk factors include longer duration of treatment, higher serum lithium concentrations, episodes of lithium toxicity, and use of other psychotropic medications. Polyuria and polydipsia may be a symptom of nephrogenic diabetes insipidus. (See "Renal toxicity of lithium", section on 'Nephrogenic diabetes insipidus'.) Clinicians should attempt to prevent polyuria at the onset of lithium treatment by [44]: ●Administering lithium once per day ●Maintaining lithium serum concentrations as low as possible ●Avoiding episodes of lithium toxicity For patients who develop polyuria, diuretics (eg, the potassium-sparing diuretic amiloride) may decrease polyuria, but caution must be used because many diuretics alter serum lithium concentrations; lithium doses may need to be adjusted and lithium levels checked more often [44]. Use of diuretics that decrease potassium levels necessitates measuring potassium levels and possibly administering potassium supplements. Specific interactions of lithium with other medications may be determined using the Lexicomp drug interactions tool included in UpToDate. Thyroid — Thyroid function is adversely affected by lithium and thus monitored with laboratory tests (see 'Laboratory tests and monitoring' above); lithium can cause goiter, hypothyroidism, chronic autoimmune thyroiditis, and possibly hyperthyroidism. The adverse effects of lithium on thyroid function and their management are reviewed separately. (See "Lithium and the thyroid".) Neither pretreatment hypothyroidism (presumably treated adequately with T4) nor lithium-induced hypothyroidism is a contraindication to lithium therapy [44,56]. Reasonable recommendations are to monitor serum thyrotropin and if it rises much above the upper value of normal, to start T4 while continuing the lithium. Consultation with an endocrinologist may also be indicated. (See "Clinical manifestations of hypothyroidism" and "Treatment of primary hypothyroidism in adults".) Parathyroid — Lithium may cause hypercalcemia, elevated serum parathyroid hormone, and hyperparathyroidism [44,50]. Patients with lithium-induced hypercalcemia and hyperparathyroidism are generally asymptomatic [57]. An elevated calcium level should prompt a serum parathyroid hormone concentration. If the hormone level is abnormal, an endocrine consult is obtained. Hypercalcemia, hyperparathyroidism secondary to lithium, and measurement of serum calcium are discussed separately. (See "Pathogenesis and etiology of primary hyperparathyroidism", section on 'Lithium therapy' and "Primary hyperparathyroidism: Diagnosis, differential diagnosis, and evaluation", section on 'Serum calcium' and "Primary hyperparathyroidism: Management".) Tremor — Lithium tremor is common; pooled results from multiple studies suggest that the prevalence is approximately 25 percent [58]. The pathogenesis may involve lithium-induced accumulation of iron in the substantia nigra [59]. Tremor secondary to lithium is classified as an action tremor, and subcategorized as an exaggerated physiologic tremor. Lithium tremors are also subclassified as a postural tremor that occurs when a specific posture, such as holding the arms outstretched or while standing, is voluntarily maintained. (See "Overview of tremor".) Onset of lithium tremor typically occurs when the drug is started or titrated up, but tremor can appear at any time during treatment [44,58]. Lithium tremor is generally symmetric, limited to the hands or upper limbs, and nonprogressive. The frequency of the involuntary rhythmic oscillation of the hands is approximately 10 Hertz. Factors that increase the risk of tremor include higher lithium doses and serum concentrations, anxiety, caffeine, medications (eg, antiarrhythmics, beta-adrenergic agents, carbamazepine, and valproate), emotional and physical stress, fatigue, and older age. The differential diagnosis of lithium tremor includes metabolic abnormalities, benign essential tremor, Parkinson disease, and lithium toxicity [58]. Tremor caused by lithium toxicity is more coarse and severe than is otherwise observed in patients treated with lithium; in addition, lithium toxicity may affect body parts other than the upper extremity, and is likely to occur with other symptoms of toxicity. (See 'Lithium toxicity' above and "Lithium poisoning", section on 'Signs and symptoms'.) Clinicians evaluating lithium tremor should obtain a history, physical examination, and laboratory tests (including a serum lithium concentration), to rule out other causes of tremor [58]. Additional information about the evaluation of lithium tremor is discussed separately. (See "Overview of tremor", section on 'Evaluation'.) Watchful waiting is a reasonable approach to lithium tremor because it often is relatively mild and resolves over time [44,58]. Management of lithium tremor that is troublesome and/or persistent starts with modifying aggravating factors (eg, decreasing caffeine intake). In addition, it may help to change the lithium preparation from long acting to short acting, or to a different salt (ie, from carbonate to citrate), or to divide the daily dose to take smaller amounts more often. For lithium tremor that still persists and causes moderate to severe functional problems, we suggest add-on pharmacotherapy (eg, beta blockers such as propranolol) [44,58]. Alternatively, the total daily dose of lithium can be reduced if feasible. Choosing and administering add-on pharmacotherapy for lithium tremor is discussed separately in the context of essential tremor. (See "Essential tremor: Treatment and prognosis".) Nausea — Nausea secondary to lithium is observed in 10 to 20 percent of patients [44]. Management strategies include the following, listed from most to least preferable: ●Taking lithium with food or after meals. ●Using a sustained release formulation of lithium (to decrease peak serum concentrations because nausea may be related to higher peak levels). ●Dividing the daily dose to take smaller amounts more often (to decrease peak serum concentrations). ●Treatment with a second drug. (See "Approach to the adult with nausea and vomiting", section on 'Antiemetics and prokinetics'.) ●Reducing the total daily dose. Nausea often abates over time, which allows patients to resume a higher dose and once daily dosing [44]. Vomiting secondary to lithium is uncommon and can indicate lithium toxicity, especially in the context of other adverse effects, such as coarse tremor and ataxia [44]. (See 'Lithium toxicity' above.) Loose stools/diarrhea — Lithium-induced loose stools/diarrhea is seen in up to 10 percent of patients [44]. Higher serum lithium concentrations (eg, greater than 0.8 mEq/L [0.8 mmol/L]) may correlate with loose stools/diarrhea; thus, management strategies include: ●Using an immediate-release formulation of lithium (to avoid distal absorption of the drug). ●Treatment with a second drug. (See "Approach to the adult with chronic diarrhea in resource-abundant settings", section on 'Symptomatic therapy'.) ●Reducing the daily dose. Weight gain — Lithium can cause weight gain through several mechanisms, such as carbohydrate craving, increased thirst, and fluid consumption, water retention related to salt retention, and reduced metabolism secondary to hypothyroidism. A meta-analysis of five randomized trials compared lithium with placebo in patients with bipolar disorder; four trials lasted to 12 or 18 months (n = 899 patients) and one trial lasted 3 months (n = 325) [50]. Clinically significant weight gain, defined as an increase >7 percent from baseline, occurred in nearly twice as many patients treated with lithium than placebo (relative risk 1.9, 95% CI 1.3-2.8). In addition, weight gain is relatively distressing, compared with other adverse effects [44]. Preventing and managing weight gain during lithium treatment is based upon nonspecific measures [44]: ●Discussing the likelihood of weight gain at the outset of treatment. ●Encouraging patients to drink low caloric drinks when thirsty. ●Dietary strategies, exercise, and drug therapy. (See "Obesity in adults: Overview of management", section on 'Drug therapy'.) ●Treating polyuria/polydipsia-induced weight gain with a diuretic, and treating hypothyroidism-induced weight gain with thyroid supplementation. (See "Renal toxicity of lithium", section on 'Treatment' and "Treatment of primary hypothyroidism in adults".) Cognitive impairment — Lithium-induced cognitive impairment appears to be one of the most distressing adverse effects of the drug and often leads to nonadherence [10,44]. Cognitive dysfunction secondary to lithium needs to be distinguished from the cognitive impairment that is associated with bipolar disorder per se, including patients who are euthymic or depressed [44,60]. Concomitant medications (eg, antipsychotics, antidepressants, and benzodiazepines) may also contribute to cognitive dulling that occurs in patients receiving lithium. In addition, it appears that the neurocognitive effects are worse at higher doses and are cumulative over time. Cognitive impairment that is due to bipolar disorder itself is discussed separately. (See "Bipolar disorder in adults: Clinical features", section on 'Neurocognitive function'.) Multiple cognitive domains are adversely affected by lithium. As an example, a meta-analysis of six studies included patients with remitted mood disorders (n = 326, primarily bipolar disorder) and found that use of lithium was associated with small to moderate impairment of immediate verbal learning and memory, creativity, and psychomotor performance [61]. However, lithium may preserve other domains of neurocognition [60]. Management strategies include dividing the daily dose to take smaller amounts more often; in addition, lowering the dose of lithium may help, because cognitive dulling may be dose related [44]. Among patients receiving polypharmacy, especially complex psychotropic regimens that include three or more drugs, discontinuing one or more drugs may improve cognitive dysfunction without exacerbating mood symptoms. Adding stimulants such as modafinil and armodafinil may possibly help, but there is no high quality evidence to support this approach. Sexual dysfunction — Lithium-induced sexual dysfunction appears to be common. One study assessed clinically stable patients with bipolar disorder who were treated with lithium for an average of 10 years (n = 100 patients; mean age 44 years); 85 percent were receiving lithium monotherapy and the mean dose in the entire sample was 800 mg/day [62]. Sexual dysfunction across multiple domains (eg, arousal, sexual drive, and penile erection/vaginal lubrication) was present in 37 percent and was associated with poor adherence. Risk factors for sexual dysfunction included older age and the presence of other adverse effects. Management strategies, beyond the general strategies for managing adverse effects (see 'General strategies' above), are discussed in the context of treating the general population of patients with sexual dysfunction. (See "Treatment of male sexual dysfunction" and "Overview of sexual dysfunction in women: Management".) Cardiac — Lithium may rarely cause cardiac dysrhythmias in patients without pre-existing cardiac disease [36,52,63]. In addition, lithium may lead to the following abnormalities on the electrocardiogram, which may anticipate the onset of dysrhythmias [52]: ●Repolarization abnormalities of the T wave or ST segment. ●Findings consistent with sinus node dysfunction. (See "Sinus node dysfunction: Epidemiology, etiology, and natural history", section on 'Other'.) ●An unmasked or modulated Brugada pattern. (See "Brugada syndrome: Clinical presentation, diagnosis, and evaluation", section on 'Provoking factors'.) These electrocardiogram findings should prompt a cardiology consult. PREGNANCY Although lithium is generally regarded as teratogenic due to increased risks of cardiac defects (eg, Ebstein anomaly) [64-66], many authorities consider the absolute risk small [67-71]. The use of lithium during pregnancy and risks of lithium exposure during pregnancy and breastfeeding are discussed separately. (See "Bipolar disorder in women: Preconception and prenatal maintenance pharmacotherapy", section on 'Refractory patients' and "Teratogenicity, pregnancy complications, and postnatal risks of antipsychotics, benzodiazepines, lithium, and electroconvulsive therapy", section on 'Lithium' and "Breastfeeding infants: Safety of exposure to antipsychotics, lithium, stimulants, and medications for substance use disorders", section on 'Lithium'.)
2 notes
·
View notes