#actinides
Photo
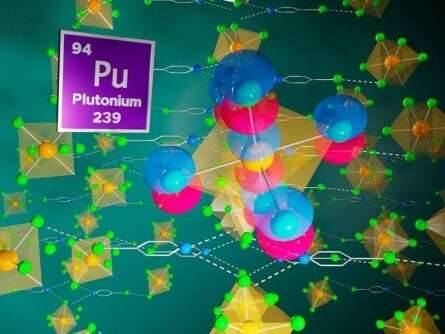
Exploring bonds and electronic structure in plutonium hybrid materials
Understanding the arrangement of electrons in compounds containing actinide elements, such as uranium and plutonium, can help advance the design of next-generation nuclear materials. These elements are challenging to study due to their complex chemistry and radioactivity. Additionally, these elements, which are in a sequence of related elements called the actinide series, have electrons that are organized in ways that don't match theoretical predictions.
Researchers have synthesized five different hybrid materials containing a particular compound subunit of plutonium and chlorine: the [PuCl6]2- anion. They probed the anion's electronic structure and found that while the electrons in the plutonium-chlorine bonds were mostly not shared (ionic), they also featured important contributions from covalent bonds where the electrons are shared. These covalent bonds correlated with the 5f shell of the quantum-mechanical model of the atom. This shell is also the region of mismatch between scientists' observations and theories of how electrons are organized around atomic nuclei.
The research is published in the journal Inorganic Chemistry.
Read more.
14 notes
·
View notes
Text

ATOMStober
Day 2. Lamp
———

Day 3. Wings
#ATOMS#Sc#scandium#transition metal#U#uranium#actinides#periodic table#chemistry#science#elements#character#illustration#digital painting#procreate#chibi art#web comics#inktober#intober2022#ATOMStober#Dreamnightober#gijinka
22 notes
·
View notes
Text
Time to do the next stage of:
I’m going to take the 10 most voted after these 3 and do a final one in a week
#what’s the most fuckable element?#what’s your favorite element?#what’s your favourite element?#tumblr polls#polls#periodic table of elements#periodic table#periodic table poll#elements#Nobel gas#alkali metals#nonmetals#actinides#mendelevium#nobelium#protactinium#plutonium#carbon#lithium#sodium#helium
4 notes
·
View notes
Text
Mamando mientras suena el cartel de santa
Black Teen Riding White Dick
Super sexy desi girl nude show
Super slut with a big ass Mia Malkova
Desi Indian Girl Blowjob her BF Outdoor Hot
Fake Taxi Big Tits Blonde Bombshell Cindy Sun Fucked in the Ass
Short Haired TS Donna A Cutie Anal Pumped To the Max
Le doy una rica mamada al amigo de mi novio
Desi bhabhi nude show
BLACK REVENGE: WHITE CUCKOLD HUMILIATION
#kilometers#tetrahydroxy#oxalic#fluework#rush#bosom#innoxious#Ruthenian#fasciculate#Rosecrans#Aria#self-gratulation#Buxton#kilovolt#pitapatation#hypohydrochloria#lunulated#Melolonthidae#Wray#actinides
0 notes
Text
You know what? I'm tired of being able to fit the entire periodic table onto a single sheet of paper. Let's put the lanthanides and actinides back where they belong. unless all of you science people are cowards, of course.
#embrace LONG TABLE#periodic table#science#chem#you can't look at it on one piece of paper?#too bad.#memorize it like a real scientist.#justice for lanthanides/actinides!
129 notes
·
View notes
Note
i saw a post abt this the other day and i have to ask. which element from the periodic table do you think is the most fuckable
I think neon would be pretty sweet and a reliable partner. But fluorine would be the kinkiest
63 notes
·
View notes
Text
Cesium-133, let it be. Cesium-134, let it be even more.
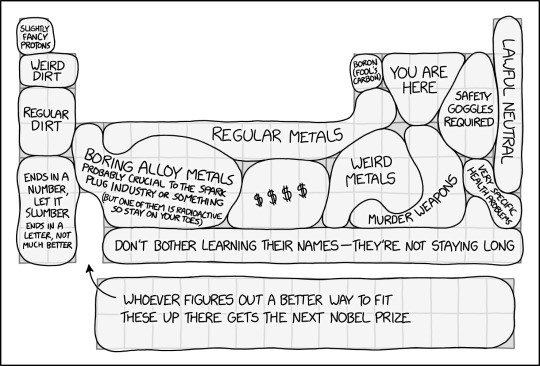
Periodic Table Regions [Explained]
Transcript
[A periodic table with regions labeled.]
[Hydrogen:] Slightly fancy protons
[Lithium and Beryllium:] Weird dirt
[Group 1 & 2 metals, Periods 3-4:] Regular dirt
[Group 1 & 2 metals, Periods 5-7:] Ends in a number, let it slumber ends in a letter, not much better
[Left side of the transition metals group:] Boring alloy metals Probably critical to the spark plug industry or something (but one of them is radioactive so stay on your toes)
[Most of the top row of the transition metals + aluminum:] Regular metals
[Below the rightmost "regular metals" - the "ordinary metals" and some transition metals:] Weird metals
[The platinum group:] $$$$
[Boron:] Boron (fool's carbon)
[Carbon, Nitrogen, Oxygen, and Phosphorus:] You are here
[The Halogens:] Safety goggles required
[Noble Gases:] Lawful neutral
[Iodine and Radon:] Very specific health problems
[Ordinary metals and metalloids - Arsenic, Antimony, Tellurium, Thallium, Lead, Bismuth, Polonium] Murder weapons
[Astatine and Period 7 from Rutherfordium onwards:] Don't bother learning their names - they're not staying long
[Lanthanides and Actinides:] Whoever figures out a better way to fit these up there gets the next Nobel Prize
8K notes
·
View notes
Text
0 notes
Text

ok smart guy 😡😡😡

og
#actinide u dipshit#dumb dumb stupid man#i love him tho#but also hate him#[🪐];; mossy’s ocs#not tagging as art#im just on a 10 hour bus ride and im kinda bored#oc shitpost
0 notes
Text
Curium [96] [Cr] [Actinide]
Curium [96] [Cr] [Actinide]
glows purple in the dark from the powerful radiation
0 notes
Photo

Using photochemistry to separate plutonium and uranium
A team of researchers at Los Alamos National Laboratory has developed a way to use photochemistry to separate plutonium and uranium—work that could make it easier to store nuclear waste. In their paper published in the journal Chemical Communications, the group describes their purification process.
In order to use plutonium in weapons or in electricity generating plants, it must first be purified. One common purification process involves the separation of plutonium and uranium. This is typically done through the use of redox agents applied during actinide separation. Afterward, chemical agents are applied to bind the actinides to allow for separation.
Unfortunately, this process creates a large amount of hazardous waste, typically in liquid form, which is stored in barrels. Storage of such waste has become politically divisive. For that reason, chemists have been searching for a cleaner way to separate plutonium and uranium that does not produce hazardous waste products. In this new effort, the team in New Mexico developed a photochemistry-based method that partly meets that specification.
Read more.
#Materials Science#Science#Photochemistry#Plutonium#Uranium#Nuclear waste#Reactions#Chemistry#Actinides#Waste
22 notes
·
View notes
Text
@hera-the-wizard
163 notes
·
View notes
Text
#polls#what’s your favourite element?#what’s your favorite element?#what’s the most fuckable element?#periodic table#periodic table of elements#periodic table poll#elements#actinides
3 notes
·
View notes
Note
There's Hydrogen and Helium
Then Lithium, Beryllium
Boron, Carbon everywhere
Nitrogen all through the air
With Oxygen so you can breathe
And Fluorine for your pretty teeth
Neon to light up the signs
Sodium for salty times
Silicon
(Phosphorus, then Sulfur) Chlorine and Argon
(Potassium) And Calcium so you'll grow strong
(Scandium) Titanium, Vanadium and Chromium and Manganese
This is the Periodic Table
Noble gas is stable
Halogens and Alkali react aggressively
Each period will see new outer shells
While electrons are added moving to the right
Iron is the 26th
Then Cobalt, Nickel coins you get
Copper, Zinc and Gallium
Germanium and Arsenic
Selenium and Bromine film
While Krypton helps light up your room
Rubidium and Strontium then Yttrium, Zirconium
Molybdenum, Technetium
(Ruthenium) Rhodium, Palladium
(Silver-war) Then Cadmium and Indium
(Tin-cans) Antimony then Tellurium and Iodine and Xenon
And then Caesium and
Barium is 56, and this is where the table splits
Where Lanthanides have just begun
Lanthanum, Cerium and Praseodymium
Neodymium's next to
Promethium, then 62's
Samarium, Europium, Gadolinium and Terbium
Dysprosium, Holmium, Erbium, Thulium
Ytterbium, Lutetium
Hafnium, Tantalum, Tungsten then we're on to
Rhenium, Osmium and Iridium
Platinum, Gold to make you rich till you grow old
Mercury to tell you when it's really cold
(Thallium) And lead then Bismuth for your tummy
(Polonium) Astatine would not be yummy
(Radon) Francium will last a little time
(Radium) then Actinides at 89
This is the Periodic Table
Noble gas is stable
Halogens and Alkali react aggressively
Each period will see new outer shells
While electrons are to the right
Actinium, Thorium, Protactinium
Uranium, Neptunium, Plutonium
Americium, Curium, Berkelium
Californium, Einsteinium, Fermium
Mendelevium, Nobelium, Lawrencium
Rutherfordium, Dubnium, Seaborgium
Bohrium, Hassium then Meitnerium
Darmstadtium, Roentgenium, Copernicium
Nihonium, Flerovium
Moscovium, Livermorium
Tennessine and Oganesson
And then we're done
Wonderful job
#rottmnt#rottmnt donnie#rise of the teenage mutant ninja turtles#rise donnie#unpause rise of the tmnt#rise of the tmnt#rottmnt leo#rise leo#disaster twins#save rise of the tmnt
45 notes
·
View notes
Photo

In 2018, a segment called “Professor E. Gadd’s Research Journal” was published on Nintendo’s official website for Luigi’s Mansion, telling the story of how E. Gadd created Gooigi.
One of the images in the journal is a periodic table, which has a minor oddity. The elements Lutetium (Lu) and Lawrencium (Lr) both appear twice on the table, both in the places they are supposed to be (main table, middle left), and also additionally at the end of the lanthanide and actinide series (bottom right).
While lutetium and lawrentium are a lanthanide and an actinide, respectively, it is standard to put them in the main table (since they are part of Group 3 together with scandium and yttrium) while relegating the rest of the series to the bottom of the table. In addition, even if the choice is made to replace the Group 3 entries in the main table with an asterisk and put the entire series on the bottom, as is done in some tables, it is not standard to have an element appear twice in the table regardless of where it is placed.
Main Blog | Twitter | Patreon | Small Findings | Source
223 notes
·
View notes
Text
Hello! I wrote a thing--mostly to get through some writer's block, but then, oops, it got a wee bit away from me!
Brave Captain Halcyon has sailed the black for quite some time, but he has known only one true foe: the vile Affini Compact, who seek to enslave Terrans at any cost…and now, Terra has surrendered. Will he--for he is definitely a "he"--be able to escape the sinister vines of Melia Actinid?
A-at least, I think that's how it went.
Maybe it was a precious little Terran, too precious to not Domesticate? Or maybe there's more to this tale that we don't know about?
27 notes
·
View notes