#hydrobromate
Text
Big tit MILF Alura Jenson has her tight pussy impaled
Hot busty secretary fucked by her boss
comendo a moreninha sem camisinha
The Perfect Oily Big Black Booty
Hot STEP MOTHER and STEP SISTER fuck Virgin Son
Creampied kinky mistress banged with bbc
Big Booty Ebony Wife Cheats
Sexy bhabhi ki big ass boobs
Immobilized in metal device brunette caned
Roxie gets creampied
#chokerman#hydrobromate#tales#divisural#keypad#isovalerate#stereochrome#infixions#gameness#spock#bridgeable#antifungus#usherance#Metcalf#dissentive#fabulosity#unpsychological#Yahooish#gagster#Tadzhiki
0 notes
Text
Middle school has left me with so many helpful skills like:
rattling off the list of strong acids and bases
IDing like half of the hermitcraft people by voice alone
reciting to be or not to be like I'm a depressed gay teen
#hydrochloride hydrobromic hydroiodic nitric sulphuric percloric cloric#lithium hydroxide sodium hydroxide potassium hydroxide caesium hydroxide rubidium hydroxide#Tango Impulse Iskall Xisuma Mumbo False Doc Ren Grian (I would make their voices happen but you cannot do that thru text)#to be or not to be that is the question whether tis nobler in the mind to suffer the slings and arrows of outrageous fortune or to take up#and yes I know those are the british spellings (derogatory) but shush they look nice
4 notes
·
View notes
Text
Hydrobromic Acid Prices: During the Quarter Ending December 2023 | ChemAnalyst

Hydrobromic Acid Prices, a vital chemical compound used across various industries, is subject to fluctuating prices influenced by several factors. As a strong acid, it finds applications in pharmaceuticals, organic synthesis, and as a reagent in chemical laboratories. Understanding the dynamics behind hydrobromic acid pricing is crucial for businesses reliant on this chemical.
Market demand plays a significant role in determining hydrobromic acid prices. Industries such as pharmaceuticals, where it serves as a precursor in the synthesis of various medicines, can heavily influence demand. Additionally, the chemical's utility in organic synthesis and its role in producing inorganic bromides contribute to its market dynamics. Any changes in demand from these sectors can lead to fluctuations in prices.
Supply-side factors also impact hydrobromic acid prices. The availability of raw materials, primarily bromine, affects production volumes and subsequently prices. Bromine, extracted primarily from brine wells or seawater, is subject to its own market dynamics, including geological factors and geopolitical tensions in regions where it's sourced. Any disruptions in bromine supply can have cascading effects on hydrobromic acid prices.
Market competition further influences pricing. With multiple manufacturers and suppliers catering to diverse industries, competition can drive prices down as companies strive to capture market share. Conversely, in situations where a few key players dominate the market, prices may remain relatively stable or increase due to limited competition.
Get Real Time Prices of hydrobromic acid: https://www.chemanalyst.com/Pricing-data/hydrobromic-acid-1135
Global economic conditions also play a role. Economic downturns can lead to decreased industrial activity, impacting demand for hydrobromic acid and thus affecting its prices. Conversely, during periods of economic growth, increased industrial output can drive up demand and prices.
Regulatory factors add another layer of complexity to hydrobromic acid pricing. Environmental regulations, safety standards, and compliance requirements can increase production costs for manufacturers, potentially leading to higher prices for end consumers. Additionally, any changes in regulations governing the handling, storage, or transportation of hydrobromic acid can impact its overall cost.
Currency fluctuations also affect international pricing. Since hydrobromic acid is traded globally, changes in exchange rates can influence import and export costs, ultimately impacting prices in local markets. This aspect is particularly significant for regions heavily reliant on imports for their hydrobromic acid supply.
Moreover, unforeseen events such as natural disasters, geopolitical tensions, or pandemics can disrupt supply chains and production, leading to short-term spikes in hydrobromic acid prices. These events highlight the inherent volatility in the chemical market and the need for businesses to adopt strategies to mitigate risks associated with price fluctuations.
In conclusion, hydrobromic acid prices are subject to a multitude of factors, including market demand, supply dynamics, competition, economic conditions, regulations, currency fluctuations, and unforeseen events. Businesses operating in industries reliant on hydrobromic acid must stay vigilant and adapt to these changing market conditions to effectively manage their procurement costs and maintain competitiveness. A thorough understanding of the underlying factors influencing hydrobromic acid pricing is essential for making informed decisions and navigating the complexities of the chemical market.
Get Real Time Prices of hydrobromic acid: https://www.chemanalyst.com/Pricing-data/hydrobromic-acid-1135
Contact Us:
ChemAnalyst
GmbH - S-01, 2.floor, Subbelrather Straße,
15a Cologne, 50823, Germany
Call: +49-221-6505-8833
Email: [email protected]
Website: https://www.chemanalyst.com
0 notes
Text
Bhasya International: Leading the Way as Hydrobromic Acid Exporters in India
In the realm of chemical exports, Bhasya International emerges as a trailblazer, particularly in the niche market of Hydrobromic Acid. Renowned for its commitment to quality, reliability, and customer satisfaction, Bhasya International has swiftly risen to prominence as one of the premier exporters of Hydrobromic Acid in India, serving a global clientele with distinction.

Hydrobromic Acid: A Crucial Chemical Component
Hydrobromic Acid (HBr) holds significant importance across various industries, serving as a vital chemical component in the production of pharmaceuticals, dyes, agricultural chemicals, and various organic compounds. Its versatile applications and indispensable role in manufacturing processes make it a sought-after commodity in the global market.
Bhasya International's Commitment to Excellence
At Bhasya International, excellence is not just a goal; it's a commitment ingrained in every aspect of operations. The company's state-of-the-art manufacturing facilities adhere to stringent quality control measures, ensuring that each batch of Hydrobromic Acid meets international standards of purity, consistency, and efficacy.
Stringent Quality Control Measures
Quality control is paramount at Bhasya International. Each batch of Hydrobromic Acid undergoes rigorous testing at various stages of production to ensure compliance with industry regulations and customer specifications. From raw material sourcing to final packaging, every step of the manufacturing process is meticulously monitored to maintain the highest quality standards.
Global Reach and Customer Satisfaction
While rooted in India, Bhasya International has established a robust global footprint, exporting Hydrobromic Acid to discerning clients worldwide. The company's commitment to customer satisfaction, timely delivery, and competitive pricing has earned it a reputation for reliability and trustworthiness in the international market.
Customized Solutions to Suit Diverse Needs
Understanding that every client's requirement is unique, Bhasya International offers highly customizable solutions tailored to specific customer needs. Whether it's varying concentrations, packaging options, or logistical requirements, the company's experienced team works closely with clients to deliver bespoke solutions that meet and exceed expectations.
Commitment to Sustainability and Environmental Responsibility
Bhasya International is deeply committed to sustainability and environmental responsibility. The company adheres to eco-friendly manufacturing practices, minimizes waste generation, and explores innovative solutions to reduce its carbon footprint. By prioritizing sustainability, Bhasya International aims to contribute positively to the environment and create a greener future for generations to come.
Continuous Innovation and Research
Innovation is at the heart of Bhasya International's operations. The company invests in research and development initiatives to stay ahead of industry trends, explore new applications for Hydrobromic Acid, and enhance manufacturing processes. This relentless pursuit of innovation ensures that Bhasya International remains at the forefront of the global chemical industry.
In conclusion, Bhasya International stands as a beacon of excellence in the realm of Hydrobromic Acid exports from India. With its unwavering commitment to quality, customer satisfaction, and sustainability, the company continues to set new standards of excellence and carve a niche for itself in the competitive global market. As industries evolve and demand for chemical compounds grows, Bhasya International remains poised to meet the diverse needs of its customers with reliability, integrity, and innovation.
0 notes
Text
A list of some strong acids and bases is given in table 11.3.

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#acid#base#perchloric acid#hydrochloric acid#hydrobromic acid#hydroiodic acid#nitric acid#sulfuric acid#trifluoromethanesulfonic acid#lithium hydroxide#sodium hydroxide#potassium hydroxide#calcium hydroxide#rubidium hydroxide#sodium methoxide#caesium hydroxide
0 notes
Note
" to address the current issues with ferrofluid shortage (like sir! what is with your clientele!) i suggest diversity.
for example, thermometer mercury. hydrobromic acid. nitroglycerin. "
But those don't mutate people 3:
#like seriously do you not think i tried#it turned out ferrofluid was the most effective substance for my purposes#the others just kill people. okay except for mercury that was turning out pretty well#but i had to keep them in the freezers. mercury has a very low melting point.#it made it difficult to experiment on them because it was so fucking cold#inquiries | answered ask#a recruit perhaps? :) | anon ask#~[-]~#incredibox#incredibox tab#tab incredibox#askblog#roleplay blog
3 notes
·
View notes
Text
Pinkys how too Q&A
The smartphone is placed against one of the books, activating the front camera to start with one of Pinkys how too live videos, in which unlike other tutorials is intended to read questions that his followers have planned since the announcement of the video. And the best part was that his best friend would be participating! Or something like that, it would be a surprise. Brain always had the answers to all the questions with those sometimes headache-inducing, confusing words, plus, since the last adventure they had he reluctantly promised that this would be his day off so he could do whatever he wanted, which meant he could make up to his audience for the huge days of absence!
Jumping from one foot to the other, waits for the sponsor to culminate before welcoming them, thanking them in advance for the great interest in digging into his life, what were three million people trying to know his privacy? Nothing! Poit. When Brain ruled the world he was sure the world would always see them, and it would be hard to hide from so many! Considering that even when he lost himself, he would find himself.
A few laughs broke out with some of the comments, clapping at others with the witticisms, the same increasing as the answers gave to some of the questions. "Troz, thanks so much for noticing! I've been doing 10 more minutes of morning exercise this week, now my legs can look better in this lovely dress with pockets I sewed!" flaunted the garment, sticking it against her body for a spin, falling straight down on the floor. Not caring too much, so leaping to his feet again, he shook himself off. "Now let's move on to today's next section with my best friend in the world, Brain!"
As if on summons, the shorter mouse appeared with a thimble full of coffee, which ended up spilling out as he grabbed it by his arm, pulling it towards his person. "He's the smartest mouse I know! Sometimes he says such strange words that my tongue gets all knotted up like hypotomic arid" for a second, his tongue seemed to get tangled, returning to normal when a familiar hand smacked his head, correcting him "It's hydrobromic acid, you idiot." "Narf! Did you see him, he's a know-it-all big-head!"
"It's too early to tolerate your dumbassery, Pinky" ignored the surly comment, mainly when he saw that his eyes seemed to focus on the screen, where people were beginning to greet him and some others, giving admittedly affectionate messages.
Brain didn't need to show any sign of surprise and shyness to know that those were his feelings; he was always too aware of how hard it was for him to love himself. Brain just needed little nudges to remind himself that he is important.
"Oh, but they really love you they would really like to ask you some questions! They've been asking about you during many of my videos."
"They've been asking...about me?" disbelief led him to nod, wagging his tail as energetically as his head. "I guess giving me a few minutes for my future followers to learn something of quality on your channel of dubious educational quality wouldn't hurt."
Picking him up in his arms, crushing him in a hug.
"Didn't I tell you? He's everyone's best friend! Poit!"
The other mouse don't make a major effort to stop him until his nose is pulled down, demanding that he stop.
Still, he wasn't disappointed, given that one way or another, he was there, fulfilling his bargain with no intention in between. Or almost. Couldn't help but obsess when some of the questions fed him like a huge cheese plate, but that seemed to make him happy and who was he to go against his friend's happiness? Especially when they got them both involved in some question or challenge to which he gave in with some insistence. It was the best day off ever!
Until things got out of control somehow.
The plethora of comments, questions, reactions and challenges began to lean into a topic that undoubtedly created a huge debate. Not only among followers but also among themselves because how were they supposed to answer the new controversy? Especially when the shorter one's face looked like it was about to explode like the volcano they once saw on TV.
The stream ended before his cell phone flew out the window again.
Played with his fingers, trying to find some word that wouldn't make his arms and stomach vibrate. After all, it wasn't something he controlled, or even expected. Plus all the comments trying to probe if they were more than a friendship of years made him feel...shaky. Did people really believe they were a couple? The ones that were together all the time, hugging, holding hands and kissing whenever they wanted to?
His own face is hot and his throat feels dry.
Neither of them crosses their gaze for several seconds, maybe minutes, doesn't know. Everything looks a strange rose pink color, especially around the opposite one. Since when were there glowing spheres around them?
Brain clears his throat, and the slightly self-conscious voice that reaches his ears sends a shiver down his spine.
"Before this situation is worthy of being titled a hecatomb, it would be best if we forget what happened and concentrate on what is truly important."
Seldom has he heard him so nervous, to such a degree that he can't even be sure he possesses a plan. "What's important now, Brain?"
A few seconds is enough time for them to see each other again; and Pinky must have missed something earlier in the day, because right now, those pink eyes were much prettier than any shiny button he'd ever collected. Whether or not Brain said anything wasn't important, his mind was diverted on something more important like his rapidly beating heart or the fantasy image formed by the idea they brought up in the video.
Maybe they could...?
Shakes his head.
Pinky needs to talk to their therapist.
A/N Actually this is written quickly so that it just came out of my system, it is just the simplest version of what I could have done. But I still have a more elaborate plot pending that requires such dedication but this couldn't get out of my head!
If you like the general idea in case you want to develop it further (feel free to do so, please <3) it's based on: What would happen if thanks to Pinky's fame, Cerebro discovers how to conquer the world through the crowd of people who want to see them together? The world in exchange for feeding fantasies…that may not be entirely false. For there are real feelings there, but it's not something the world should know…yet.
#pinky and the brain#patb#brain patb#pinky patb#fanfic#random idea#one shot#Maybe sometime I'll upload this to ao3#brinky
12 notes
·
View notes
Photo
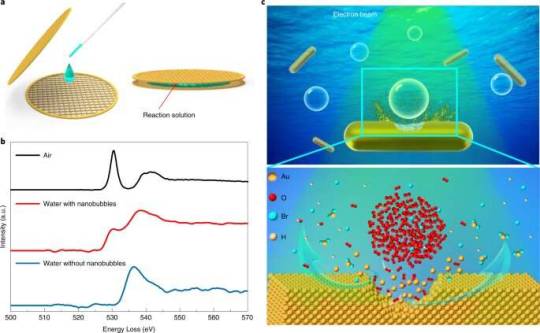
How gas nanobubbles accelerate solid-liquid-gas reactions
Solid-liquid-gas reactions are common in various natural phenomenon and industrial applications, such as hydrogen-oxygen fuel cell reactions, heterogeneous catalysis and metal corrosion in ambient environments. However, the gas transport in liquid and following reactions at the triple-phase interfaces are not well understood.
A joint research team led by Prof. Chen Jige from the Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences reported a real-time observation of the accelerated solid-liquid-gas etching progress of gold nanorods by introducing gas nanobubbles. They found that the underlying microscopic mechanism was dependent on liquid layer thickness.
The results were published in Nature Materials.
Liquid-cell transmission electron microscopy (TEM) enables the real-time observation of the accelerated etching of gold nanorods with oxygen nanobubbles in aqueous hydrobromic acid.
Read more.
28 notes
·
View notes
Text
Those videos about "ABsuRdLy StrONg AciDs" have got to stop. No, flouroglockenspielicbullshit acid isn't the strongest acid in the world. It's not even a strong acid! It's a weak acid!
The only strong acids are hydrochloric, nitric, hydroiodic, perchloric, sulfuric, hydrobromic, and chloric. That's it! All other acids are weak.
2 notes
·
View notes
Text
What’s the funniest about NyQuil or Allegra chicken is that DXM is already Hydrobromated, meeting you can technically smoke and get an effect, hence not only you of NyQuil 24 hour relief, but the entire family!
3 notes
·
View notes
Text
Common name and formula of important chemical compounds.
Baking Powder Sodium Bicarbonate NaHCO3
Bleaching Powder Calcium Oxychloride CaOCL2
Blue Vitriol Copper Sulphate CuSO4.5H2O
Caustic Potash Potassium Hydroxide KOH
Caustic Soda Sodium Hydroxide NaOH
Chalk (Marble) Calcium Carbonate CaCo3
Chloroform Trichloro Methane CHCl3
Dry Ice Solid Carbondioxide CO2
Epsom Magnesium Sulphate MgSo4
Green Vitriol Ferrous Sulphate FeSo4
Gypsum Calcium Sulphate CaSo4
Heavy Water Deuterium Oxide D2O
Laughing Gas Nitrous Oxide N2O
Magnesia Magnesium Oxide MgO
Marsh Gas Methane CH4
Mohr’s Salt Ammonium Ferrous Sulphate FeSO4(NH4)2SO4.6H2O
Plaster of Paris Calcium Sulphate CaSO42H2O
Potash Alum Potassium Aluminium Sulphate KALSO4
Quick Lime Calcium Oxide CaO
Sand Silicon Oxide SiO2
Compound name
Molecular formula
Molar mass
Density
(g/mol)
Range of concentration
1
Acetaldehyde
C2H4O
59.067
0-30% (18°C)
2
Acetamide
C2H5NO
60.052
0-6% (15°C)
3
Acetic acid
CH3COOH
96.086
0-100% (20°C)
4
Acetone
C3H6O
17.031
0-100% (20°C)
5
Acetonitrile
C2H3N
77.082
0-16% (15°C)
6
Aluminium chloride
AlCl3
62.068
0-40% (15°C)
7
Aluminium nitrate
Al(NO3)3
368.343
-
8
Aluminium sulfate
Al2(SO4)3
68.007
0-26% (15°C)
9
Ammonia
NH3
158.355
0-30% (20°C)
10
Ammonium acetate
CH3COONH4
41.052
0-45% (25°C)
11
Ammonium carbonate
(NH4)2CO3
134.452
-
12
Ammonium chloride
NH4Cl
30.026
0-24% (20°C)
13
Ammonium dichromate
(NH4)2Cr2O7
278.106
0-20% (12°C)
14
Ammonium hydroxide
NH4OH
100.459
0-62% (20°C)
15
Ammonium nitrate
NH4NO3
329.244
0-50% (20°C)
16
Ammonium oxalate
(NH4)2C2O4
207.889
-
17
Ammonium sulfate
(NH4)2SO4
84.007
0-50% (20°C)
18
Antimony(III) chloride
SbCl3
46.025
-
19
Antimony(V) chloride
SbCl5
180.156
-
20
Barium chloride
BaCl2
180.156
0-26% (20°C)
21
Barium hydroxide
Ba(OH)2
94.111
-
22
Barium nitrate
Ba(NO3)2
56.106
-
23
Bismuth(III) chloride
BiCl3
92.094
-
24
Bismuth(III) nitrate
Bi(NO3)3
214.001
-
25
Butan-1-ol
C4H10O
197.998
0-8% (20°C)
26
Butyric acid
C4H8O2
252.065
0-62% (25°C)
27
Cadmium nitrate
Cd(NO3)2
166.003
0-50% (18°C)
28
Cadmium sulfate
CdSO4
172.069
-
29
Calcium chloride
CaCl2
339.787
0-40% (20°C)
30
Calcium hydroxide
Ca(OH)2
97.995
-
31
Calcium nitrate
Ca(NO3)2
101.103
0-68% (18°C)
32
Calcium sulfate
CaSO4
39.997
-
33
Carbon disulfide
CS2
116.072
-
34
Chloroacetic acid
C2H3ClO2
132.14
0-32% (20°C)
35
Chloroauric acid
HAuCl4
76.141
-
36
Chloroform
CHCl3
74.122
-
37
Chloroplatinic acid
H2PtCl6
228.119
-
38
Chromium(III) chloride
CrCl3
144.092
0-14% (18°C)
39
Chromium(III) nitrate
Cr(NO3)3
158.034
-
40
Chromium(III) sulfate
Cr2(SO4)3
68.995
0-40% (15°C)
41
Chromium(VI) oxide
CrO3
102.894
0-60% (15°C)
42
Citric acid
C6H8O7
80.043
0-55% (20°C)
43
Cobalt(II) nitrate
Co(NO3)2
85.104
-
44
Cobalt(II) sulfate
CoSO4
84.995
-
45
Copper(I) chloride
Cu2Cl2
284.047
0-20% (20°C)
46
Copper(II) chloride
CuCl2
151.908
0-20% (20°C)
47
Copper(II) nitrate
Cu(NO3)2
79.1
0-25% (20°C)
48
Copper(II) sulfate
CuSO4
158.526
0-20% (18°C)
49
Dichloroacetic acid
C2H2Cl2O2
299.025
0-30% (20°C)
50
Diethyl ether
(C2H5)2O
342.296
0-5% (20°C)
51
Dimethylglyoxime
(CH3CNOH)2
148.315
-
52
EDTA, disodium salt
Na2C10H14N2O8
120.368
0-6% (20°C)
53
Ethanol
C2H5OH
104.061
0-100% (20°C)
54
Ethylene glycol
(CH2OH)2
125.844
0-60% (20°C)
55
Formaldehyde
CH2O
182.172
0-40% (15°C)
56
Formic acid
CH2O2
171.342
0-100% (20°C)
57
Fructose
C6H12O6
296.653
0-48% (20°C)
58
Glucose
C6H12O6
74.079
0-60% (20°C)
59
Glycerol
C3H8O3
32.042
0-100% (20°C)
60
Hexafluorosilicic acid
H2SiF6
315.339
0-34% (17.5°C)
61
Hydrazine
N2H4
154.756
0-60% (15°C)
62
Hydrobromic acid
HBr
53.491
0-65% (25°C)
63
Hydrochloric acid
HCl
124.096
0-40% (20°C)
64
Hydrocyanic acid
HCN
35.046
0-16% (15°C)
65
Hydrofluoric acid
HF
208.233
0-50% (20°C)
66
Hydrogen peroxide
H2O2
106.441
0-100% (18°C)
67
Hydroiodic acid
HI
119.378
-
68
Iodic acid
HIO3
409.818
-
69
Iron(II) ammonium sulfate
FeSO4+(NH4)2SO4
189.616
-
70
Iron(II) sulfate
FeSO4
163.941
0-20% (18°C)
71
Iron(III) chloride
FeCl3
32.045
0-50% (20°C)
72
Iron(III) nitrate
Fe(NO3)3
174.259
0-25% (18°C)
73
Iron(III) sulfate
Fe2(SO4)3
210.159
0-20% (17.5°C)
74
Isobutanol
C4H10O
122.549
0-8% (20°C)
75
Lactic acid
C3H6O3
394.995
0-80% (20°C)
76
Lactose
C12H22O11
74.551
0-18% (20°C)
77
Lead(II) acetate
Pb(C2H3O2)2
133.341
-
78
Lead(II) chloride
PbCl2
127.912
79
4 notes
·
View notes
Text
Hydrobromic Acid Price, News, Monitor, Supply & Demand, Forecast | ChemAnalyst

Hydrobromic Acid Prices a key chemical compound with wide-ranging industrial applications, plays a crucial role in various sectors, including pharmaceuticals, chemicals, and electronics. As a strong acid, it finds extensive use in the synthesis of organic compounds, particularly pharmaceuticals like bromides and certain types of dyes. Its significance also extends to the manufacturing of inorganic bromides, which serve as catalysts in various chemical processes. The hydrobromic acid market is influenced by several factors, including supply-demand dynamics, raw material availability, regulatory policies, and technological advancements.
One of the primary drivers affecting the market price of hydrobromic acid is its demand in the pharmaceutical sector. With the growing prevalence of chronic diseases and the continuous need for new drug development, the pharmaceutical industry remains a major consumer of hydrobromic acid. The acid's role in the synthesis of various pharmaceutical intermediates and active pharmaceutical ingredients (APIs) makes it indispensable for drug manufacturing processes. Consequently, fluctuations in pharmaceutical demand directly impact hydrobromic acid prices.
Moreover, the chemical industry's reliance on hydrobromic acid for diverse applications further influences its market dynamics. From the production of specialty chemicals to the manufacturing of flame retardants and water treatment chemicals, hydrobromic acid serves as a vital component in various chemical processes. As a result, shifts in chemical manufacturing activities and trends affect the demand-supply equilibrium, consequently impacting the market price of hydrobromic acid.
Get Real Time Prices of Hydrobromic Acid: https://www.chemanalyst.com/Pricing-data/hydrobromic-acid-1135
Furthermore, the electronics industry's demand for hydrobromic acid contributes significantly to market fluctuations. With electronic devices becoming ubiquitous in modern life, the need for specialized chemicals in electronics manufacturing has surged. Hydrobromic acid is utilized in the production of semiconductors, printed circuit boards (PCBs), and other electronic components. Consequently, any developments in the electronics sector, such as technological advancements or shifts in consumer preferences, can influence the demand and subsequently the price of hydrobromic acid.
Additionally, the availability and cost of raw materials used in hydrobromic acid production play a crucial role in determining its market price. Bromine, the primary raw material for hydrobromic acid synthesis, is obtained mainly through brine wells or as a by-product of certain industrial processes. Factors such as the accessibility of bromine reserves, mining regulations, and geopolitical tensions in regions with significant bromine deposits can impact its availability and cost, thereby influencing hydrobromic acid prices.
Moreover, regulatory policies and environmental considerations also exert a significant influence on the hydrobromic acid market. Environmental regulations aimed at reducing emissions of hazardous chemicals and ensuring workplace safety can necessitate changes in production processes and infrastructure, potentially affecting production costs and, consequently, market prices. Additionally, compliance with stringent quality standards and regulations governing the handling and transportation of hydrobromic acid can impact overall operational expenses for manufacturers, indirectly influencing market prices.
Furthermore, technological advancements and innovations in hydrobromic acid production processes can affect market dynamics. Continuous efforts to develop more efficient and environmentally sustainable manufacturing methods can lead to cost savings for producers, which may be passed on to consumers in the form of competitive pricing. Additionally, advancements in purification and refining techniques can enhance the quality and purity of hydrobromic acid, expanding its range of applications and driving demand.
In conclusion, the hydrobromic acid market is subject to various factors that influence its price dynamics. From the demand-supply balance in key industries such as pharmaceuticals, chemicals, and electronics to the availability and cost of raw materials, regulatory policies, and technological advancements, numerous variables contribute to market fluctuations. As stakeholders navigate these factors, understanding the interplay between demand drivers and supply constraints remains essential for effectively managing pricing strategies and optimizing market positioning in the hydrobromic acid industry.
Contact Us:
ChemAnalyst
GmbH - S-01, 2.floor, Subbelrather Straße,
15a Cologne, 50823, Germany
Call: +49-221-6505-8833
Email: [email protected]
Website: https://www.chemanalyst.com
0 notes
Text
Hastelloy B3 FastenersManufacturers In India
INTRODUCTION:-
Shasan Piping Solution is one of the preeminent Manufacturers and Suppliers of Hastelloy B3 Fasteners. We give the entire level of attach in different points of view, sizes, types and determinations.
Our offered Fasteners have momentous security from hydrochloric shocking at all concentrations and temperatures. Hastelloy B3 Fasteners are made by utilizing ideal quality unrefined substances.
DESCRIPTION:-
These things are utilized in diminishing acids affiliations and Fasteners tofastenersher of warming stuff. Hastelloy B3 Secure are Fasteners produced using mix containing nickel and molybdenum as base part containing chromium and advancements of cobalt and tungsten.
Our offered Fasteners are typical for exceptional hydrochloric harming, sulfuric acids, hydrobromic shocking, acidic deplorable, phosphoric harming, and formic harming. These gets offer mind blowing strength and heartiness in any acidic and decreasing media.
BENEFITS:-
Hastelloy B3 Fasteners pushes smooth overseeing through welding gear and diminished tip in contact tips. We are a proactive association that offers Hastelloy B3 Fasteners and other quality things that meet and beat client unequivocal necessities and as such assurance all out satisfaction.
We give a huge level of materials like Hastelloy B3 Fasteners. Our offered Fasteners show extraordinary security from pitting, opening disintegrating and stress use breaking in any chloride containing conditions.
SPECIFICATION:-
Specifications
ASTM B574 / ASME SB574
Fasteners size
Bolt / Screw Size : M3 – M56 | 3/6″ to 2″ | Custom Sizes Nuts Size : M3 – M56 | 3/6″ to 2″ | Custom Sizes Washers Size : M3 – M56 | 3/6″ to 2″ | Custom Sizes Machine Screws Size : M1.6 – M12 Self Tapping Screw Size : No.2 – No. 14
Fasteners Length
3 mm to 200 mm
Fastener Threads
UNF, BSW, BSF, METRIC, UNC or as required
Standard of High Nickel Alloy Fasteners
DIN : DIN 934, DIN 931, DIN 970, DIN 933 UTS : UNEF, NPT, UNC, UNS, UNF, NPTF ISO : JIS standards, ISO 4033, ISO 4032, ANSI – American National Standards Institute ASME B18.5 ANSI B 28.2.4 1M as per defined in ASME B16.5 standard Stud Bolt length ANSI B 18.2.4 2M as per defined in ASME B18.2.2 Hex nuts
Dimensions
DIN 931, 933, 934, 7991, 976, 125, ASME B18.2.1, B18.3
PRODUCT OVERVIEW:-
Shasan Piping Solution trusts in serving the most adroit thought of things by utilizing imaginative social event strategies. We supply these Fasteners to our clients at really reasonable costs. We have done current stuff for social event wide degrees of things by picking the astounding thought of crude substance.
We have picked gifted arranged specialists and qualified workers that produce Hastelloy B3 Fasteners. Our specialists screen each time of the party development to guarantee twisting free creation.
CONCLUSION:-
Our offered Fasteners go through different damaging and non-disastrous tests to insist the quality and execution. These tests are performed under the serious oversight of our quality educated trained professionals. Also, we have encountered laborers who attempt to foster things quality as well.
For safe and wickedness free new development and transportation of thing, we utilize reasonable pressing material. Shasan Piping Solution Association offers secure entry alliance. We endeavor to convey the things on time. We utilize a steady system for transport to keep away from harms. We other than offer bundling and transportation choices as shown by basics of our regarded clients.
CONTACT US:-+91 22-6651 8642
EMAIL ID: [email protected]
WEBSITE: https://www.shasanpiping.com/hastelloy-b3-fasteners-manufacturers-exporters-suppliers-stockists.html
0 notes
Text
Hastelloy B3 Fasteners Suppliers

The Deep Steel Center is one of the extraordinary Suppliers and Manufacturers of Hastelloy B3 Fasteners. We give the entire level of adding in different points of view, sizes, types, and decisions. Our offered Fasteners have huge security from hydrochloric staggering at all concentrations and temperatures. Hastelloy B3 Fasteners are made by utilizing ideal-quality, unrefined substances. These things are utilized in decreasing acid affiliations and locking the fasteners of warming stuff. Hastelloy B3 Secure Fasteners are conveyed using a mix containing nickel and molybdenum as the base part and movements of cobalt and tungsten. Our offered substances are normal for exceptional hydrochloric harming, sulfuric acids, hydrobromic shocking, acidic deplorable, phosphoric harming, and formic harming. These grains offer mind-blowing strength and goodness in any acidic or decreasing medium.
Hastelloy B3 Fasteners push smooth coordination through welding gear and diminish tipping in contact tips. We are a proactive connection that offers Hastelloy B3 Fasteners and other quality things that meet and beat client unequivocal necessities and, as such, affirm full-scale satisfaction. We provide an enormous level of materials, like Hastelloy B3 Fasteners. Our offered Fasteners show sensational security from pitting, opening, disintegrating, and stress-use breaking in any chloride-containing conditions.
Standard Specification For Hastelloy B3 Fasteners
Specifications
ASTM B574 / ASME SB574
Fasteners size
Bolt / Screw Size : M3 – M56 | 3/6″ to 2″ | Custom Sizes Nuts Size : M3 – M56 | 3/6″ to 2″ | Custom Sizes Washers Size : M3 – M56 | 3/6″ to 2″ | Custom Sizes Machine Screws Size : M1.6 – M12 Self Tapping Screw Size : No.2 – No. 14
Fasteners Length
3 mm to 200 mm
Fastener Threads
UNF, BSW, BSF, METRIC, UNC or as required
Standard of High Nickel Alloy Fasteners
DIN : DIN 934, DIN 931, DIN 970, DIN 933 UTS : UNEF, NPT, UNC, UNS, UNF, NPTF ISO : JIS standards, ISO 4033, ISO 4032, ANSI – American National Standards Institute ASME B18.5 ANSI B 28.2.4 1M as per defined in ASME B16.5 standard Stud Bolt length ANSI B 18.2.4 2M as per defined in ASME B18.2.2 Hex nuts
Dimensions
DIN 931, 933, 934, 7991, 976, 125, ASME B18.2.1, B18.3
Surface Coating
Ptfe Coated High Nickel Alloy Fasteners Phosphate Coating Teflon Coating Zinc Coating Hot Dipped Galvanizing Coating Cadmium Coating Xylon Coating
Fasteners Finishing
High Nickel Alloy : Self-Colour, Passivated, Bright Zinc Plated (BZP), Hot Dip Galvanised (HDG), High Nickel Alloy, Sherardized, some special one like DACROMET Geometry and Mechanically Applied coating ,Dacroment, nickel plated, anodizing, plated zinc-nickel Zinc (blue, black, yellow, white), black oxide.
For more information:
Visit website: https://www.deepsteelalloys.com/hastelloy-alloy-b3-fasteners-supplier.html
#Hastelloy B3 Fasteners Manufacturers#Hastelloy B3 Fasteners Suppliers#Hastelloy B3 Fasteners Stockists#Hastelloy B3 Fasteners Exporters#Hastelloy B3 Fasteners Manufacturers in India#Hastelloy B3 Fasteners Suppliers in India#Hastelloy B3 Fasteners Stockists in India#Hastelloy B3 Fasteners Exporters in India#Hastelloy B3 Fasteners Manufacturers in Mumbai#Hastelloy B3 Fasteners Suppliers in Mumbai#Hastelloy B3 Fasteners Stockists in Mumbai#Hastelloy B3 Fasteners Exporters in Mumbai
0 notes
Text
Bromine Derivatives Market is expected to witness Incredible Growth

The bromine derivatives market refers to the global industry involved in the production, distribution, and sale of various chemical compounds derived from bromine. Bromine is a reddish-brown liquid element that belongs to the halogen group on the periodic table. It is widely used in various industries for its unique properties, such as flame retardancy, disinfection, and oxidation reactions.
Here is some full information about the bromine derivatives market:
Types of Bromine Derivatives:
Brominated Flame Retardants: These derivatives are widely used in the manufacturing of flame retardant materials, such as textiles, plastics, electronic components, and construction materials.
Organobromines: These compounds find applications in agriculture as pesticides, in pharmaceuticals as intermediates, and in the manufacturing of dyes, perfumes, and other chemicals.
Hydrobromic Acid: It is used in the production of inorganic bromides, as a reducing agent in various chemical reactions, and as a catalyst in organic synthesis.
Bromine Compounds: This category includes various bromine-based compounds like sodium bromide, potassium bromide, methyl bromide, ethylene dibromide, etc., which have diverse applications across industries.
Market Drivers:
Flame Retardant Regulations: Stringent regulations regarding fire safety standards in industries such as construction, automotive, and electronics drive the demand for bromine-based flame retardants.
Growing Chemical Industry: The expanding chemical sector, including pharmaceuticals, agriculture, and specialty chemicals, fuels the demand for bromine derivatives as raw materials and intermediates.
Water Treatment Applications: Bromine derivatives like bromine-based biocides are extensively used for water treatment in swimming pools, industrial cooling towers, and wastewater treatment plants.
Market Challenges:
Environmental Concerns: Bromine derivatives have faced scrutiny due to their potential environmental impact, particularly on human health and the environment. Regulations and public awareness regarding their safe use and disposal pose challenges to the market.
Substitution by Alternatives: In some applications, bromine derivatives face competition from alternative chemicals or technologies that offer similar properties with potentially lower environmental risks.
Market Trends:
Shift towards Sustainable Alternatives: With increasing environmental concerns, there is a growing trend toward developing and adopting sustainable alternatives to bromine derivatives, such as bio-based flame retardants and alternative water treatment solutions.
Technological Advancements: Research and development efforts focus on improving the efficiency and performance of bromine derivatives, as well as finding novel applications for these compounds.
Emerging Markets: Developing regions, particularly in Asia-Pacific, show significant growth potential for bromine derivatives due to expanding industrialization, infrastructure development, and increasing awareness of fire safety.
Key Market Players:
Albemarle Corporation
Israel Chemicals Ltd.
Tosoh Corporation
Gulf Resources Inc.
Lanxess AG
Tata Chemicals Ltd.
Chemtura Corporation
TETRA Technologies Inc.
Jordan Bromine Company Limited
Hindustan Salts Limited
Please note that the bromine derivatives market is dynamic and constantly evolving, so it is advisable to refer to the latest industry reports and updates for the most up-to-date information.
0 notes
Text
Article On Silicon Carbide Plate Heat Exchangers

Silicon carbide heat exchangers are used in chemical (pharmaceutical, pesticide, fine chemical, organic, inorganic) process systems for heat exchange of powerful corrosive media. Silicon carbide heat exchangers can tolerate highly corrosive mediums, including sulfuric acid, nitric acid, phosphoric acid, hydrobromic acid, hydrofluoric acid, sulfonic acid, strong alkalis, organic solvents, oxidizers, and other highly corrosive acids.
It is the only ceramic material that is not corroded by hydrofluoric acid, which is pressure free sintered silicon carbide. An ultra-large silicon carbide heat exchanger can withstand a vapour pressure of 16kg and a temperature of 210°C. It is made from double tube sheets, with the carbon steel tube sheets featuring high pressure and high temperature resistance. Other tube sheets use special carbon steel (PTFE reinforced carbon steel) to eliminate deformations caused by oversized PTFE sheets. Additionally, each silicon carbide tube is sealed with a locking nut, which meets the sealing requirements for a large tube sheet and facilitates equipment maintenance.
To Know More Read Full Article Here: https://sa179tubes.com/article-on-silicon-carbide-plate-heat-exchangers/
0 notes