Text
Activity 8:
1. Choose any Teacher-Submitted Activity from any of the Chemistry Simulations (http://phet.colorado.edu/en/simulations/category/chemistry ) that we have not yet explored in this course through Activities 1-7 and post your results/data and/or answers.
For this, I decided to look into the Balloons and Buoyancy simulation, since I quite like balloons, and it was something I found to be interested. I chose Patrick Foley’s “Gas Relationships and Buoyancy” activity, and attached are the sheets I filled out.



2. Work with any of the Chemistry Simulations to create your own For Teachers activity and post on your blog.
The criteria for this is as follows:
a. must identify and meet three (3) Wisconsin Standards for Science (WSS) standards.
b. must be original work
c. must be scientifically accurate and appropriate for the directed grade level.
I’m hoping to become a teacher for preschool and/or kindergarten children. Therefore, I would want to keep ideas and concepts to a basic understanding so that we can build on them later. I chose to use the balloon and static electricity simulation on the website because I know that I was enamored with balloons as a child, and it’s important to involve both learning and play when children are that young. Granted, we probably wouldn’t be using a simulation like this when they’re in preschool, but this activity could still work for 1st-3rd graders.
Step 1. I would fill up a balloon and ask all the kids to gather around me and watch my experiment.
Step 2. I would rub the balloon on my hair and pull it away, showing that my hair follows it. It’d rub it on my hair again and let go, and show that the balloon sticks to my hair.
Step 3. I would ask what the children think causes this.
Step 4. I would then go on to say that this is called static electricity. “Static electricity is the buildup of electrical charge in an object. So when you’re shuffling on a carpet in your socks and you touch something and it shocks you? That’s static electricity. When you put on a hat in the winter and you take it off and your hair stands on end? Static electricity. Static electricity happens when objects have opposite charges - positive and negative - and are attracted to each other because of it. When one object rubs against another, this electrical charge can be created. Some materials stick more than others because they conduct electricity better - wool (like your shirt) and hair get staticy more easily when rubbed against a balloon than some other objects might.”
Step 5. After explaining, I would get them into groups of 3 or so and give them a balloon.
Step 6. I would then ask the children to find an object in the room to rub the balloon on and see if it sticks.
Step 7. I would then ask a couple of kids to line up with their socks and shuffle across the carpet and see if they can shock me.
Step 8. Once they’ve all had a taste of what static electricity is, I would sit them down and show them the simulation. I would show how the cross means it’s positive, and the line means its negative. I would run the balloon across the shirt and I would show how all the negative signs get transferred to the balloon, and how every time I move the balloon away from the shirt now, it wants to get back to those positive signs.
This activity would cover these WSS standards:
SCI.CC1.K-2
Students recognize that patterns in the natural and human designed world can be observed, used to describe phenomena, and used as evidence.
SCI.SEP1.A.K-2
Students ask simple descriptive questions that can be tested. This includes the following: Ask or identify questions that can be answered by an investigation.
SCI.SEP6.A.K-2
Students use evidence and ideas in constructing evidence-based accounts of natural phenomena. This includes the following: use information from observations (firsthand and from media) to construct and evidence-based account for natural phenomena.
3. Explore the course set of student blogs https://www.tumblr.com/blog/vizchemwinterm2019 and answer the question, "which freezes faster, hot water or cold water?" Your answer should be based on data from your experiments and other student's experiments. Also explore the practice of formulating a hypothesis. Did you and your classmates formulate a hypothesis that you then wanted to prove correctly? As I look through the blogs, it is clear that there are competing hypotheses.
From looking at the other students’ blogs and their hypotheses for the question, “Which freezes faster, hot water or cold water?” it appears as though there are indeed competing hypotheses. Some said that they believed that the cold water would freeze faster because it was closer to freezing temperature already (like I believed in my experiment), while others believed that it would be the hot water that would freeze first, either because of the Mpemba effect or because of expansion. Some hypotheses were stated with exact measurements, others as more general statements, but each hypothesis seemed to want to be proven. If I hadn’t already, and I came across these hypotheses, I would want to test them to see which hypothesis was correct.
3 notes
·
View notes
Text
Activity 7:
1. Review the Content Slides Acids and Bases on the D2L site.
2. At the pH Scale PhET simulation, complete the For Teachers activities “pH Scale inquiry-based intro to acid-base” posted by Trish Loeblein on the pH Scale simulation at PhET (http://phet.colorado.edu/en/simulation/ph-scale). Post the answers with your scientific explanations from the “Clicker_questions_pH_Scale.pdf ” posted by Trish.
1. The color of a solution identifies if it is an acid, base, or neutral solution.
Answer: B. False. It’s not the color that identifies a base/acid/neutral solution, but the pH level.
2. Which solution is basic?
Answer: D. More than one. Both B and C are basic because their pH levels are over 7.
3. Which solution is acidic?
Answer: C. It has more hydronium ions (H3O+) that make it acidic than hydroxide ions (OH-) which would make it basic.
4. Which solution is basic?
Answer: B. It has more hydroxide ions than hydronium ions.
5. Which solution is acidic?
Answer: D. More than one: both A and B are acidic because of the Hydronium levels
6. How will adding water affect the pH?
Answer: A. It will increase the pH. This is because adding the water to the acidic solution will dilute the concentration of H+ ions, and so the pH will increase.
7. How will equal amount of water affect the pH?
Answer: B. It will decrease the basicity. This is because as you dilute a solution, it will become more like pure water, which has a pH of 7.
8. What is the order from most acidic to most basic?
Answer: A. A B C
9. What is the order from most acidic to most basic?
Answer: E. C A B
10. If spit has a pH = 7.4, what does that tell you about the water equilibrium?
Answer: A. Something was added that made the equilibrium shift left. The pH is more than 7, so something had to shift it to the basic side of the equilibrium.
3. At the Acid-Base Solutions PhET simulation, complete the For Teachers activities “Strong and Weak Acids” posted by Kelly Lancaster, Laurie Langdon on the Acid-Base Solutions simulation (http://phet.colorado.edu/en/simulation/acid-base-solutions) and post on your blog your data and answers to the questions posed in their exercise, "Acid_CU_GenChem2_Activity ".
(Sorry for the orange answers: Made it a different color to stand out when typed, then printed it to draw on and took a picture to upload, but it’s a little hard to read on here. Click and zoom in to read it more clearly.)

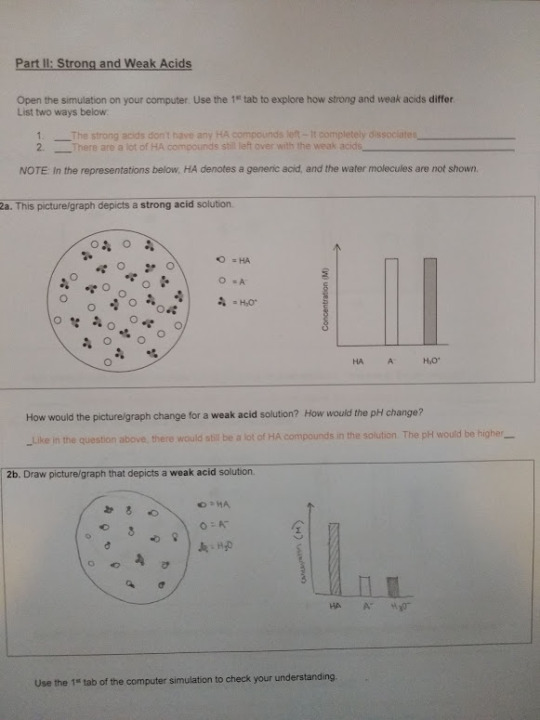
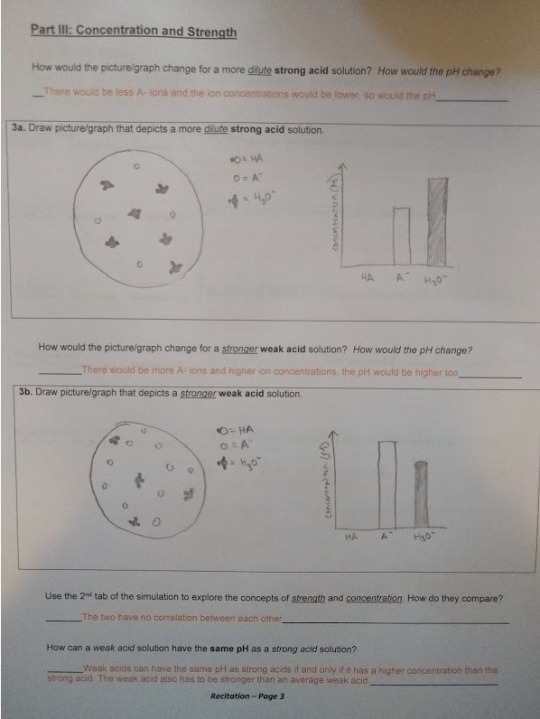

4. Post the common name, chemical name and chemical structure for a common acid and base that you commonly use.
Lemon Juice - Citric acid - H3C6H5O7 - Acid
Baking Soda - Sodium bicarbonate - NaHCO3 - Base
0 notes
Text
Activity 6:
1. Convert 0°F, 32°F, 70°F, and 212°F to Kelvin
0°F=255.37 K, 32°F=273.15 K, 70°F=294.26 K, 212°F=373.15 K
2. Under the For Teachers area of the State of Matter webpage, complete the Teacher Submitted Activities: States of Matter Simulation Lab by Kelly Vaughan. Complete the lab worksheet as if you were a student, and then post your results.




3. In the States of Matter simulation, choose the Solid, Liquid, and Gas Tab at the top of the screen. Choose the water molecule and cool the water to 0 K. Describe how the water molecules are aligned and attracted to each other. Which atoms are attracted to which other atoms?
They’re aligned with the hydrogen atoms bonding to the oxygen atoms, but not if there are 3 hydrogens on the oxygen atom already. They’re aligned in a hexagonal pattern, and are completely still.
4. Discuss why this statement is not true for water: As a liquid freezes, the molecules come closer together and have greater attraction for each other.
As a liquid freezes the molecules don’t come closer: they’ve just slowed down enough so that their attractions to one another can arrange them into more fixed positions.
5. Switch to the Phase Changes Tab on the States of Matter simulation. Notice how on the bottom right there is a small red dot that indicates where the system is at as far as temperature, pressure and state of matter. Play with the simulation to notice changes, notice that when you push down the pressure can go way up and explode the box. On your blog, report a temperature and pressure required to make oxygen a liquid. This is sometimes how the oxygen exists in pressurized oxygen tanks, perhaps like ones you may use to go diving.
55 ATM pressure, 100 K
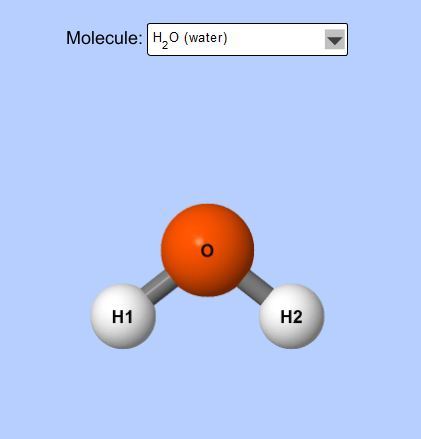
6. Switch to the Molecule Polarity simulation and go the the Real Molecules tab. Post an image of a molecule that is considered polar and an image of a molecule that is non-polar.
Polar = H2O
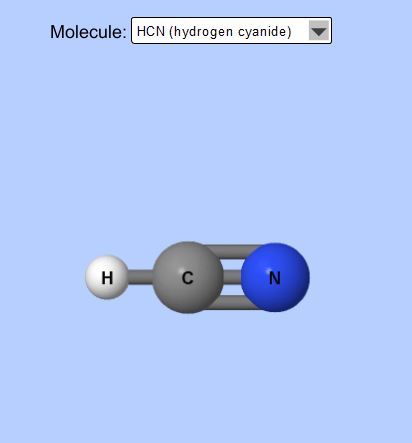
Nonpolar = HCN
7. In Activity 1, students explored the addition of salt to water to affect the freezing point. In terms of polarity, why are salt ions easily dissolved in water? At the molecular level, why does the addition of salt prevent the formation of ice at certain temperatures?
In terms of polarity, NaCl is a polar molecule. The reason that it dissolves so well in water is because polar molecules dissolve polar molecules - the positive part of the water molecules attracts the negative chloride ions, and the negative part of the water molecules attracts the positive sodium ions. It balances out, and the two become one.
When you add salt to ice, it lowers the freezing points of that water and causes it to melt. That water then becomes salty water, and any ice that is in contact with that water also becomes salty, making its freezing point lower and melting it as well. Liquid water also allows the ions in the salt to separate into the positively charged sodium and negatively charged chlorine, which in turn react with the water molecules. This reaction gives off heat because these bonds are more stable than the ions are by themselves, and that heat then thaws the ice.
8. Identify and describe at least two Wisconsin Standards for Science (WSS) that this activity addresses.
SCI.CC1.m
Students recognize macroscopic patterns are related to the nature of microscopic and atomic-level structure. They identify patterns in rates of change and other numerical relationships that provide information about natural and human-designed systems. They use patterns to identify cause and effect relationships and use graphs and charts to identify patterns in data
SCI.CC3.3-5
Students recognize natural objects and observable phenomena exist from the very small to the immensely large. They use standard units to measure and describe physical quantities such as mass, time, temperature, and volume.
0 notes
Text
Activity 5:
1. Run the Build an Atom simulation http://phet.colorado.edu/en/simulation/build-an-atom and build a neutral lithium atom and a neutral boron atom. Take a picture, or a screen shot, of these two atoms. List the number of protons, neutrons and electrons for each. Also look up and post the density for each of the elements.

Lithium: P=3, N=4, E=3. Density=0.534 g/cm^3

Boron: P=5, N=6, E=5. Density=2.34 g/cm^3
2. Define density and the equation for density.
Density is defined as “the degree of compactness of a substance” or “the ratio between mass and volume or mass per unit volume - how much stuff an object has in a unit volume.” The equation is d=M/V.
3. Run the Density simulation http://phet.colorado.edu/en/simulation/density and complete one(your choice) of the prepared For Teachers activities on the simulations page and post your results. The activity you choose should be one of the student intended activities.
I decided to use this activity: https://phet.colorado.edu/en/contributions/view/3307

4. Complete the Mystery Blocks activity on the Density simulation. Post the data you collected (mass, volume, and density) and the identification of the material and the known density.
Mystery block activity:

5. Identify and post the Science Standards (could be Wisconsin or Next Generation Science Standards) that could be met through these activities completed in Activity 5
Because there is more than one possible solution to a problem, it is useful to compare and test designs.
I believe this one works because we didn’t know why certain objects float while others don’t, so we ran an experiment that zeroed out all the possibilities and figured out the solution to the problem.
Scientists use evidence to explain the natural world.
This one works too because I went through multiple parts of my simulation in order to figure out why it was that certain objects float while others don’t.
2 notes
·
View notes
Text
Activity 4:
1. Describe what is meant by Three Dimensional Learning.
Three Dimensional Learning refers to the three pillars that support each standard. These three pillars or dimensions are: Science and Engineering Practices, Crosscutting Concepts, and Disciplinary Core Ideas. Three Dimensional Learning must teach in a way that includes all of these dimensions. As an example, instead of just teaching the basic concepts of something, it must also teach how the practices and core ideas are worked into experiments and observations. It is difficult to fully understand one without the others.
2. How are the Wisconsin Standards for Science (WSS) connected to other disciplines such as math and literacy?
They hold similar principles. Basically, the state wants to have a general, common template for educators to base the way they teach around. The state isn’t trying to get everyone to each exactly the same curriculums or anything like that, but they are trying to make sure that each teacher/educator holds the same ideas on the principles of certain subjects. Math and Literature both have Core Standards for different age/grade levels that educators must teach towards.
Also, each of the WSS can more or less be directly translated into math and literature. You need to know them all together, and it’s difficult to understand one without the other. For example: in math, you can know the idea that 2 times 2 is 4, but without knowing the practice of how to get there or the concept of what exactly multiplication is, you won’t fully understand why 2 times 2 is 4. Same with Literacy: you may be able to copy writing a word down, but without knowing how to sound it out or what the word means, you’ll have no idea what the word actually is.
3. Go to the Google Doc: Standards document split up by specific elementary grades instead of grade bands and access the Grade 2 page of the Google Doc and under column D (Performance Indicator) describe something that you have done either in this class or outside of this class, perhaps in previous classes, that indicates that you have met the Performance Indicator. Each of these descriptions should be at least a paragraph long. There are many for the Engineering, Technology, and the Application of Science category, so choose only three of those Performance Indicators for your blog.
Life Science:
Animals obtain food they need from plants or other animals. Plants need water and light.
In previous classes, I learned about the food chain and how energy passes from being to being throughout it. Plants get their energy from the sun in a process called photosynthesis, and through water and nutrients in the dirt. Some animals then eat those plants in order to get their energy. Then, those animals are eaten by other, bigger animals so they can get their own energy. This cycle continues until an animal dies, and it’s body decomposes into the soil, turning into nutrients for the plants.
Physical Science:
Pushes and pulls can have different strengths and directions, and can change the speed or direction of an object’s motion, or start or stop it. A bigger push or pull makes things speed up or slow down more quickly.
This is a process known as force. The bigger the force exerted on an object, the faster the object will go. I learned about this in a past class by experimenting with pushing different sized weights. More force is needed to push a heavier object to get it to move, and less is needed to push a lighter object and get it to move. The reason for this is because the force needed to make an object move is equal to the mass times the acceleration.
When objects touch or collide, they push on one another and can result in a change of motion.
This can be explained by Newton’s third law: when one object exerts a force on a second object, the second object exerts an equal-and-opposite force on the first object. Therefore, if one object pushes on another object, or two objects collide with one another, they have different reactions. I did experiments in the past with blocks: say a block slams into another, stationary block on a table. The first block would either slow down or stop, but the second block would move in the same direction the first one was. Say that the same thing happened, but two blocks moved towards each other at the same speed - they would collide and end up stopping because the forces exerted on each other would cancel each other out and they would stop moving.
Bigger pushes and pulls cause bigger changes in an object’s motion or shape
This is true. I explained it more above, but basically the more force exerted on an object, the more affected the object would be. If you push a ball harder, the ball is going to roll faster. If you push a swing harder, the swing is going to swing higher. If you pull a drawer harder, the drawer is going to come out faster. In previous classes, we focused on how these forces were exerted, and would find the resistance (if there was any).
Sunlight warms Earth’s surface
In previous classes, I learned that we should look at the Earth’s atmosphere like it’s one giant greenhouse. A greenhouse is used to keep plants warm and sheltered and cozy during cold times. The sun comes in and warms the ground, and then the roof doesn’t allow that heat to leave. The gasses in Earth’s atmosphere (specifically carbon dioxide) let sunlight in during the day and it heats up the ground. But at night when there isn’t any sun, the ground releases the warmth back into the air and space, but the gasses keep some of it in so that we don’t all freeze.
Earth and Space:
Weather is the combination of sunlight, wind, snow or rain, and temperature in a particular region and time. People record weather patterns over time.
I had multiple activities in different classes that related to weather patterns. Most of these activities pertained of recording weather patterns from different areas over time and comparing them to other areas. Different areas have different climates, which means different weather patterns take place, which means that different environments occur. Very dry, very hot expanses of land like a dessert are not likely to be found in very humid, very wet places like the Amazon Rainforest. Britain is known to be rainy and overcast, California is known to be sunny and hot, Antarctica is known to be very cold and snowy. Depending on your place in the world, different weather patterns arise.
Plants and animals can change their local environment.
This is true in many instances. In previous classes, we learned about migratory patterns of birds. These birds could change their environment by leaving when it got to be a sort of environment they didn’t like (too hot or too cold) and going to a place that has the environment they did like. Also, introducing foreign species into an ecosystem is generally not a good thing. For example: we learned about people dumping their goldfish into lakes to “set them free,” but those goldfish grew to be very big, and would eat up important organisms that may clean the water, or that may be an important food supply for another species. Then the entire energy cycle goes out of whack, all because one small thing was changed.
Engineering, Technology, and the Application of Science:
Because there is more than one possible solution to a problem, it is useful to compare and test designs.
This was made evident in this class when many people had different ways of setting up the experiment in Activity 1. Each of us was trying to figure out the answers to the same questions, but we all came up with different ways to get there. By following through with multiple different possible ways to get a solution, it actually is easier to know whether or not your answer in the end is correct. If we all did the exact same experiment, we might end up missing something that we would have missed if we all tried different techniques and compared our end results.
Science and Engineering involve the use of tools to observe and measure things.
Over the entirety of my science experience, we’ve used tools to observe and measure. In Activity 1 I needed a thermometer and a measuring cup/spoon for just about everything. When learning about biology I would often need a microscope to be able to view living cells and bacteria. When experimenting with chemicals you need gloves and eye protection sometimes breathing masks. Sometimes you need to use simulations on a computer in order to truly understand how something works that maybe you wouldn’t be able to create an experiment out of otherwise. Tools help us to truly be able to observe and measure the world around us in ways that we wouldn’t be able to by ourselves.
Designs can be conveyed through sketches, drawings, or physical models. These representations are useful in communicating ideas for a problem’s solutions to other people.
Without designs, many experiments would be difficult, if not impossible, to pull off correctly. In previous classes, some of the moving parts of the experiment were unknown enough to us that, without the diagrams of step by step instructions, it would have taken far too long for us to understand what the activity was meant to teach us. In other classes, we were meant to create sketches of our findings to show what we did in our experiment so that others would be able to recreate it. In this class, I created graphs in order to show my data in Activity 1. I also made physical diagrams of atom bonds out of olives and feta cheese in Activity 3. Diagrams, designs, sketches, or any models are extremely important to get your ideas across.
0 notes
Text
Activity 3: part 2.5
2. Post an image from the web, the chemical systematic (IUPAC) name, common name, and the molecule formula for 20 chemicals that you use or eat (continued)
Acetic acid - Acetic Acid - Vinegar - CH3COOH

Magnesium Hydroxide - Magnesium Hydroxide - Antacid - Mg(OH)2

Sodium Hypochlorite - Sodium Hypochlorite - Bleach - NaOCl

Ethanol - Ethanol - Alcohol - C2H5OH

Aluminum Chlorohydrate - Aluminum Chlorohydrate - Antiperspirant - Al(n)Cl(3n-m)(OH)m

Calcium carbonate - Calcium Carbonate - Chalk - CaCO3

Magnesium Sulfate - Magnesium Sulfate - Epsom Salt - MgSO4

Aspartame - N-(L-α-Aspartyl)-L-phenylalanine, 1-methyl ester - Artificial sweetener - C14H18N2O5

Silicon Dioxide - Silicon Dioxide - Sand - SiO2

Ascorbic acid - (5R)-[(1S)-1,2-Dihydroxyethyl]-3,4-dihydroxyfuran-2(5H)-one - Vitamin C - C6H8O6

3. Look over your molecules and the bonding characteristics, how many bonds does each of the following elements typically have? Carbon? Hydrogen? Oxygen?
Carbon usually forms 4 bonds, Hydrogen usually forms 1 bond, and Oxygen usually forms 2 bonds
4. What does IUPAC stand for?
IUPAC stands for the International Union of Pure Applied Chemistry
5. As you explore ingredients, notice how everything around us is made up of chemicals consisting of atoms bound together into molecules. But what about companies that claim their products are chemical free! How can this be? Here is an example:
http://www.naturalhealthcareproducts.com/Cleaning-Products.php
Do a little web searching and propose what chemicals are actually in this product. Keep in mind, that everything at the molecular level is a chemical, whether it be made in nature or in a lab.
Technically, there is nothing that is completely “chemical free.” Chemicals aren’t necessarily a bad thing though; chemical compounds make up most of what we use and consume in our day-to-day lives. Upon looking at the website for this product, even some of the things that it boasts are “chemical free” are in fact chemicals: the “chlorine scavenger” may get rid of chlorine, but it is actually a modified, liquid sodium bisulfite formulation. A “Scavenger” itself is a chemical substance added to a mixture in order to remove or de-activate impurities and make sure no unwanted reactions occur. So, no, it’s not entirely chemical free.
6. Also do a little searching on the web and share on your blog how many chemicals are typically found in things like coffee, milk, beer or whiskey? Only need to choose one.
Apparently, milk is a cocktail of all sorts of chemicals. Many people believe that milk is very good and wholesome for you, and it is - in its raw form. However, after it’s been through the pasteurization process and is put into containers and eventually makes its way to your glass, it is far from pure. When tested, medications like anti-inflammatories (niflumic acid, mefenamic acid, ketoprofen, diclofenac, phenylbutazone, naproxen, flunixin, diclofenac), antibiotics (forfenicol), Natural hormones (estrone), sex hormones (17-beta-estradiol), steroid hormones (17-alpha-ethinylestradiol), anti-malaria drugs (pyrimethamine), and antifungal drugs (triclosan). Some of these existed already in the milk producer’s body (human or not), but some of them were added via an outside source. Some dairy farms routinely give drugs to their cows, even though high levels of added hormones and antibiotics is illegal. Also, one doesn’t want high levels of those things in your milk, since they’re actually quite harmful to your health.
1 note
·
View note
Text
Activity 3: part 2
2. Post an image from the web, the chemical systematic (IUPAC) name, common name, and the molecule formula for 20 chemicals that you use or eat
Ammonia (Cleaner) - IUPAC name Azane - Common Name Ammonia Windex - Molecule formula NH3

Acetone - Propan-2-one - Nail polish remover - (CH3)2CO

Sodium Chloride - Sodium Chloride - Salt - NaCl

Glucose - D-glucose - Sugar - C6H12O6

Amoylose - Glucopyranan - Cornstarch - C6H10O5

Hydrogen Peroxide - Hydrogen peroxide - Antiseptic - H2O2

Sodium Bicarbonate - sodium hydrogen carbonate - baking soda - NaHCO3

Salicylic acid - Hydroxybenxoic acid - Asprin - C7H6O3

Propane - Propane - Propane/Fuel - C3H8

Monosodium Glutamate - Aminopentanedioate - MSG - C5H8NO4Na

0 notes
Text
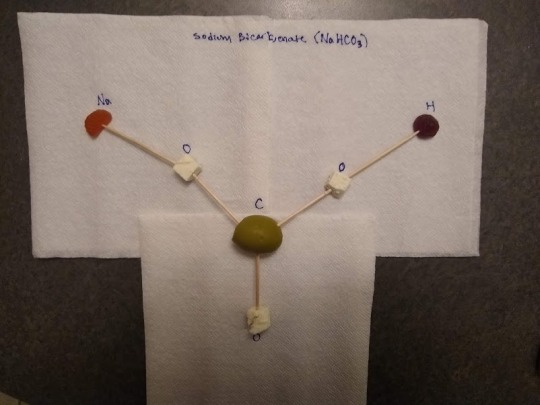
Activity 3: part 1
1. Post a picture of three 3-dimensional Ball and Stick molecular models(choose your three favorite molecules) that you have created with common items around your home. Also post a molecular structure image(image from the web, of either a Kekule Structure or a Ball and Stick Model) and the IUPAC name of the molecule.
(I used green olives, feta cheese, vitamin gummies, and toothpicks)
Water (H2O) - Oxidane


Carbon dioxide (CO2) - Carbon Dioxide

Sodium bicarbonate (NaHCO3) - Sodium Hydrogen Carbonate
0 notes
Text
Activity 2:
Pennies = Protons
Quarters = Neutrons
Dimes = Electrons
Helium

Hydrogen

Boron

1. What is the atomic number for each of your models?
Helium: 2
Hydrogen: 1
Boron: 5
2. What is the atomic mass number for each of your models?
Helium: 4.002602
Hydrogen: 1.00794
Boron: 4.002602
3. In your models, which two subatomic particles are equal in number?
The protons and Electrons are the same
4. How would you make an isotope for one of your models? What would change with the model?
I would have to add neutrons to the model, which would end up changing the atomic mass.
5. Considering the overall volume of your element models, what makes up most of the volume of an atom?
The majority of the volume of an atom is taken up by the space in between the rows of moving electrons - over 99 percent is empty space, in fact.
6. For one of your models, show with another image what happens when energy excites an electron.
Boron

Excited Boron

One of boron’s electrons moved to a higher energy level.
7. Once the electron is excited, what do we typically observe when the electron returns to the ground-state?
When an excited electron returns to a ground state, it lets out a photon of energy, which can be seen by us as light.
8. Why are some elements different colors when they are excited?
When you heat an atom, some of its electrons are excited to higher energy levels, but according to the Bohr model electrons have different allowed energy levels - the more electrons, the more energy. When an electron drops from one level to a lower energy level, it releases a photon of energy that can be seen as light. Therefore, when electrons drop from different high energy levels to lower energy levels, their emitted lights are different because the energy released is different.
9. You may observe fireworks over the New Year’s, explain how the different colors of fireworks arise.
Fireworks are made with a time-delay fuse that ignites a tube containing gunpowder and causes a reaction. The heat excites the electrons in the specific elements also inside the elements, and as the electrons go back to their ground state, the excess energy releases as light. Different elements release different colored lights, so they’re carefully chosen when the fireworks are made so that the firework produces the desired effect.
10. Explain the overall organizational structure of the periodic table.
The elements are arranged from left to right and top to bottom in order of increasing atomic number and coinciding atomic mass. Vertical columns in the periodic table are called Groups or families and contain elements with similar chemical properties. Horizontal rows in the periodic table are called Periods and have a range of properties such as metallic on the left and nonmetallic on the right.
11. List two example elements for each of these groups or classes: Alkali Metals, Alkaline Earth, Halogens, Noble Gases, Transition Metals, Non-Metals, and Metalloids.
Alkali Metals: Potassium, Rubidium
Alkaline Earth: Calcium, Radium
Halogens: Fluorine, Iodine
Noble Gases: Helium, Argon
Transition Metals: Titanium, Copper
Non-Metals: Hydrogen, Oxygen
Metalloids: Boron, Silicon
12. Paragraph that describes how electrons behave:
They behave oddly, to say the least. Electrons behave as both matter and as waves. According to the video, electrons had the ability to pass through two slits and create two stripes, acting like matter would if put through the same experiment. It also had the ability to pass through two slits and create an interference pattern, just like waves.The only difference between these two experiments was that it only created two stripes when an observer was present. Electrons act of their own accord, and even quantum physicists are baffled by what they do.
0 notes
Text
Activity 1: part 3
3. Does salt water freeze faster or slower than regular water
Hypothesis: If ½ tablespoon of salt is added to ½ cup of water, then it will take longer to freeze than the same amount of water without salt
Controlled Variables:
Amount of water (½ cup)
Glasses
Water temp
Independent Variable(s):
Salt/no salt
Dependent Variable(s):
How long the water takes to freeze
Materials:
Water (½ cup for each glass)
Thermometer
Timer/stopwatch
Liquid measuring cup
Glasses
Salt
½ tablespoon
Tape
Marker

Procedure:
1. Fill up two glasses with ½ cup of water in each
1a. Be sure the temperature of both is the same
2. Add ½ tablespoon of salt to one of the glasses and mix until salt is dissolved
3. Label the glass with salt “salt” and the one without “no salt”
4. Put in the freezer and check in periodically (every 5-10 minutes or so) with stopwatch until a layer of ice can be seen that covers up the entire surface of the water


Observations:

Data:

Theory:
As my data shows, my hypothesis was correct: adding salt to water makes it take longer to freeze. This is because adding salt to water lowers its freezing point - that doesn’t mean that it isn’t cold, it just means that the water won’t turn into a solid until it reaches even colder temperatures, which means it takes longer to freeze than regular water does. We add salt to roads in order to melt the ice and create a less slippery and dangerous environment to drive in.
This experiment is quite repeatable: it’s a simple experiment that just about anyone can do with simple materials easily found around the house. The only thing with this experiment that makes it slightly more difficult is the time aspect - if you don’t have a lot of time on your hands to sit and wait while your water freezes multiple times, then this isn’t the sort of repeatable experiment for you.
Overall Averages:
Hot water freeze time: 40.97 min
Cold water freeze time: 28.95 min
Hot water boil time: 1.15 min
Cold water boil time: 2.46 min
Salt water freeze time: 62.12 min
Regular water freeze time: 29.33 min
The majority of the science concepts that this experiment covered were about water and it’s different states of matter (which isn’t all that surprising). For example, I watched as the water turned from liquid into solid form in both the freezing experiments, and watched it evaporate into steam and turn into a gas in the boiling experiment. I learned a little bit about water molecules when I learned about how the Mpemba Effect stretches the molecules apart. I also used heat and temperature in my experiments to determine whether or not they affected freezing rates. Basically, this experiment taught the basics of how to conduct a lab experiment, run it yourself, and recognize key introductory ideas of chemistry.
In my experiment, I tested to see if hot or cold water would freeze faster, if hot or cold water would boil faster, and if adding salt would affect water’s freeze time. To do this, I separated each component of the experiments into controlled and independent/dependent variables to know what I should and shouldn’t change. Next, I planned out a procedure that made sense to me and followed it through. Finally, I timed and recorded the results. My results show that cold water freezes faster (though, by the Mpemba Effect, hot water should), hot water boils faster, and salt water freezes more slowly than not-salted water.
A couple real world examples for this information are as follows: I always wanted to know if there was a way to freeze ice more quickly. So, now I know that if I want ice cubes faster, I’ll use hotter water instead of colder water. Same goes for boiling water: get the water hot before you go to boil it so that it takes less time to boil - always good to have that pasta ready sooner rather than later! And finally, I already knew that putting salt down on the roads got rid of the ice, but that’s a real-world application that we all use and rely on in the winter months to keep our roads clean and safe (especially in Wisconsin).
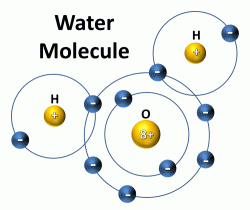
Above is a representation of a water molecule, and below is a link to a youtube video about how the water molecules work together when in different states of matter.
https://www.youtube.com/watch?v=dHJmOH38agY
2 notes
·
View notes
Text
Activity 1: part 2
2. Does hot water or cold water boil faster?
Hypothesis: If ½ cup of water is already hot when put into a pot on a stove, then it will take less time to boil than if the water started cold because it’s already closer to boiling temperature.
Controlled Variables:
Amount of water (½ cup)
Pot water was boiled in
Stove setting
Independent Variable(s):
Original water temp
Dependent Variable(s):
How long the water takes to boil
Materials:
Water (½ cup for each experiment)
Thermometer
Timer/stopwatch
Liquid measuring cup
Pot/Pan
Stove

Procedure:
1. Measure out ½ cup of water (cold)
2. Record temperature of water
3. Turn on stove
4. Pour water into pot
5. Put pot on stove
6. Time until water boils
7. Repeat steps 1-6 with cold water 2 more times, then 1-6 again but with hot water

Observation:

Data:

Theory:
My hypothesis that hot water would boil faster was correct. The reasoning behind this is that hot water is already closer to the boiling temperature, and takes less energy and time to get there. Cold water absorbs heat faster, but once it reaches the point that the hot water started at, it begins to slow down, therefore making its boiling process longer than the hot water.
1 note
·
View note
Text
Activity 1: part 1
1. Does hot water or cold water freeze faster?
Hypothesis: If two glasses with ½ cup of cold and hot water respectively are placed into a freezer, then the glass with cold water will take less time to freeze than hot water because it is already closer to freezing temperature.
Controlled Variables:
Amount of water (½ cup)
Glasses
Independent Variable(s):
Original water temp
Dependent Variable(s):
How long the water takes to freeze
Materials:
Water (½ cup for each glass)
2 Glasses (preferably similar if not the exact same sort)
Thermometer
Tape/pen (to label)
Timer/stopwatch
Liquid measuring cup
Freezer

Procedure:
1. Turn sink tap to coldest setting, measure out ½ cup of water and put in a glass
2. Check temperature of water
2a. Record temp
3. Label glass as “Cold”
(CONCURRENTLY)
4. Turn sink tap to hottest setting, measure out ½ cup of water and put in a glass
5. Check temperature of water
5a. Record temp
6. Label glass as “Hot”
7. Put both glasses in the freezer and set a stopwatch, checking in periodically (every 5-10 minutes or so) until a layer of ice covers the entire surface of the water
8. Repeat steps 1-7 two more times



Observation:

Data:
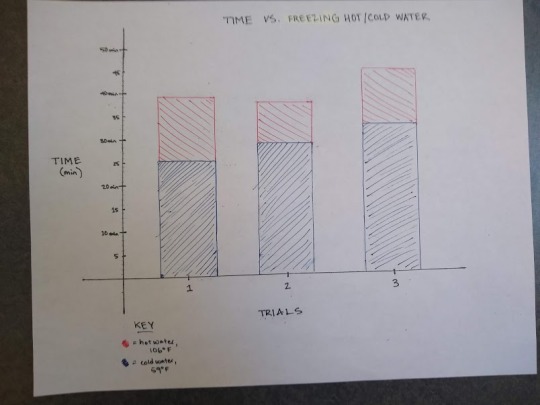
Theory:
According to my data, my hypothesis was correct: it takes longer for hot water to freeze than cold water. However, after doing some research online, I realized that the hot water should have taken less time to freeze than the cold due to the phenomena known as the “Mpemba Effect.” The Mpemba Effect has a few different explanations as to why it happens, but the most compelling to me was that warm water evaporates more rapidly and, since it’s an endothermic process, it cools the water down and freezes it more quickly. One thing I noticed about the hot water freezing that didn’t happen with the cold water in my experiment was the ice crystals on the sides of the glass, indicating that the water had been evaporating and was frozen to the side of the glass which is why this explanation makes sense to me. The Mpemba Effect is also explained by the hydrogen bonds bringing water molecules into close contact, forcing the O-H bonds to stretch and store more energy (This is the website I found: https://medium.com/the-physics-arxiv-blog/why-hot-water-freezes-faster-than-cold-physicists-solve-the-mpemba-effect-d8a2f611e853 )
My data was correct to my experiment, but didn’t correctly reflect the results it was supposed to. There’s plenty of room for error in this experiment that may have caused it: perhaps I waited too long to put them in the freezer when I was trying to gauge their temperatures at the same time, or maybe (the most likely possibility, I think) I didn’t make the water hot enough to start with, so it didn’t have enough energy to evaporate to cool itself down fast enough.
3 notes
·
View notes