#pteropus alecto
Text

Black flying foxes (Pteropus alecto) hang from a tree in Kununurra, Western Australia
by John Anderson
#black flying fox#megabats#bats#pteropus alecto#pteropus#pteropodidae#chiroptera#mammalia#chordata#wildlife: australia
494 notes
·
View notes
Text

Black flying fox (Pteropus alecto) in south-east QLD, Australia
#black flying fox#pteropus alecto#flying fox#bats#fruit bat#biology#zoology#australia#animals#australian animals#ecology#conservation#fauna#nature
8 notes
·
View notes
Text
Randomly making a list of bat species that I feel the most connected to! Reminder; this definitely isn't all of them, as I'm a bat ambitherian and there are 1,400+ species of chiroptera, and the only bat I don't feel connected to is the Hammerhead Bat. This starts from the most connected. This is separate from my physical nonhumanity as a werebat because there I have no set species.
🦇 Common Vampire Bat (Desmodus rotundus)
🦇 Eastern Red Bat (Lasiurus borealis)
🦇 Hoary Bat (Lasiurus cinereus)
🦇 Spectral Bat (Vampyrum spectrum)
🦇 Spectacled Flying Fox (Pteropus conspicillatus)
🦇 Black Flying Fox (Pteropus alecto)
🦇 Northern Yellow Bat (Lasiurus intermedius)
🦇 Northern Ghost Bat (Diclidurus albus)
Can't figure out where to put V
🦇 Painted Bat (Kerivoula picta)
And more! Those haven't been ranked yet. ^^
24 notes
·
View notes
Text

Young black flying fox bat
The black flying fox or black fruit bat (Pteropus alecto) is a bat in the family Pteropodidae. It is among the largest bats in the world, but is considerably smaller than the largest species in its genus, Pteropus. The black flying fox is native to Australia, Papua New Guinea, and Indonesia. It is not a threatened species.
📷 Ken Drake
97 notes
·
View notes
Note
Do you see bats a lot in Australia or Cali? They're some of my favorite animals :)
They just want to eat bugs and/or pollinate and find a nice dark place to sleep the day away.
There are a few different bat species where I live, but the ones we usually have to gently remove from buildings with a net or see flying around after sunset are big brown bats, or Eptesicus fuscus.
I've only ever seen little brown bats, Myotis lucifugus, in California. In Australia, especially in the Northern Territory near Kakadu, the bat I see most are the black flying fox (Pteropus alecto) and the little red flying fox (Pteropus scapulatus). They're everywhere. Really awesome little guys (little relative to me--they're massive bats).
3 notes
·
View notes
Text
Giving a bat flowers might preempt a pandemic
Giving a bat flowers might preempt a pandemic
A black flying fox (Pteropus alecto) takes flight in an urban flying fox roost in Queensland, Australia. | Image: Pat Jones
Stressed, hungry bat populations are linked to growing cases of an emerging zoonotic disease in Australia, new research finds. The bats have learned to adapt to more persistent food shortages by roosting closer to humans. That raises the risk of the potentially fatal Hendra…

View On WordPress
0 notes
Text
Today I found bat wings.
Just the wings, off a big monkey-sized bat, lying in a park - they were very black so I’m guessing Pteropus alecto?
rest of bat: missing. I’m guessing eagle?
1 note
·
View note
Photo

🦇Bat Fact! Do you know of the Black Flying-Fox (Pteropus Alecto)? These blossom bats are the largest species of flying-fox in Australia, coming in at 500-1000g in size and with flight speeds of 35-40kmh! This bat has a wingspan of more than 1m! Named after their striking black fur, these bats are found in Northern and Eastern Australia within forests and woodlands, as well as in Papa New Guinea and Indonesia. These bats are vulnerable to loss of their feeding areas due to habitat loss. This bat is listed as “Least Concern” by the IUCN🦇
#batfacts #bats #bat #akhyls #education
📸Photo by G.B. Baker/Australian Museum📸
⬇️Follow Bat Facts⬇️
https://akhylsthebat.tumblr.com/
https://www.minds.com/akhylsthebat/
https://twitter.com/AkhylsBatFacts
https://www.facebook.com/groups/137858924078846/
https://t.me/AkhylsBatFacts
Disclaimer: All images used here are for educational purposes and are not used in any way for profit or to promote any products or services. Copyright Disclaimer under section 107 of the Copyright Act 1976, allowance is made for “fair use” for purposes such as criticism, comment, news reporting, teaching, scholarship, education and research. Fair use is a use permitted by copyright statute that might otherwise be infringing
6 notes
·
View notes
Link
So many cute batties!
#Photography#Animal#Mammal#Bat#Flying Fox#Chiroptera#Yinpterochiroptera#Pteropodidae#Pteropodinae#Pteropus#Pteropus poliocephalus#Grey Headed Flying Fox#Pteropus alecto#Black Flying Fox#Tal'ngai Dha'run
1 note
·
View note
Link
Bats are reservoirs for a large number of viruses which have potential to cause major human disease outbreaks, including the current coronavirus disease 2019 (COVID-19) pandemic. Major efforts are underway to understand bat immune response to viruses, whereas much less is known about their immune responses to bacteria. In this study, MR1-restricted T (MR1T) cells were detected through the use of MR1 tetramers in circulation and tissues of Pteropus alecto (Pa) bats. Pa MR1T cells exhibited weak responses to MR1-presented microbial metabolites at resting state.
0 notes
Photo

Why do bats have so many viruses?
By Rachel Ehrenberg
July 15, 2020
For several weeks in March, Arinjay Banerjee would eat breakfast at 6 a.m. and then drive the empty roads of Toronto to a restricted-access lab. Then he’d ready himself for work, donning three layers of gloves, a helmeted mask kitted with an air-purifying respirator and a surgical-style gown.
The stringent conditions in that Toronto lab — only one level below the most secure in the biosafety hierarchy — were crucial. Banerjee, a virologist, was on a team working to isolate SARS-CoV-2, the virus that causes covid-19, from one of the first patients in Canada so that they could get a jump on vaccine development.
Banerjee was the bat guy. He had expertise in isolating dangerous pathogens. And he’d studied how bats interact with viruses like the one that causes the Middle East respiratory syndrome, one of hundreds of coronaviruses that the mammals can harbor.
Bats have become newly infamous as reservoirs of deadly viruses. In addition to hosting an ancestral version of MERS, which has caused repeated outbreaks in people, bats also harbor very close relatives of the virus that caused the 2003 severe acute respiratory syndrome outbreak and today’s novel coronavirus pandemic. They are the suspected reservoir of the Ebola virus and natural hosts for Hendra, Nipah and Marburg viruses — all of which can be deadly in people.
But despite the long list of bat-dwelling viruses, the animals don’t seem to be bothered by their many invisible inhabitants. Scientists want to know why.
Today, a growing number of them suspect that the key lies in special features of the bat immune system — ones that spark responses to viral invasion that are very different from what goes on in humans.
“It’s very intriguing,” Banerjee says. “I wake up thinking about it every day. Why do bats have this immune response that’s so different from ours and so different from other mammals?”
Of course, many viruses exist in wildlife, often causing little harm to their natural hosts and only making trouble for us when they jump to human beings or other creatures with which they don’t share a long evolutionary history. Ducks and other water birds muck about while carrying myriad strains of influenza A; pigs aren’t fazed by hosting hepatitis E.
But bats appear to be special, if only in the number of high-profile viruses that they carry and appear to tolerate. With few exceptions — including rabies and the more obscure Tacaribe virus — when bats get infected with viruses they
“They can remain in good health and display no discernible signs of disease,” says Raina Plowright, an infectious-disease ecologist and wildlife veterinarian at Montana State University in Bozeman.
Before covid-19, scientists already were piecing together some of the peculiarities of the bat-virus relationship. That research has taken on new urgency, and it raises an intriguing possibility. If we better understand how bats tolerate their viral passengers, it might point to treatments that could make human infections less severe.
“Rather than trying to reinvent the wheel, we could learn from what evolution has developed in a bat, where the outcome is not disease but it’s something that enables survival upon infection with a particular virus,” says cellular immunologist Judith Mandl of McGill University in Montreal. “If we figure that out, then maybe we can apply the same principles and modulate the immune response in humans.”
When a host, whether bat or human, is infected with a pathogen, the ensuing interaction is often described as a battle. But there’s a growing appreciation of the importance of disease tolerance, a “keep calm and carry on” approach by the immune system, which limits damage to the host but doesn’t worry about getting rid of every trace of a pathogen.
Although many details are missing — there are some 1,300 bat species, and studies typically focus on one or a handful — recent studies suggest that such tolerance captures how bats interact with many of the viruses they carry.
First, the bats mount a speedy but nuanced offensive that stops the virus from multiplying with abandon. Second, and perhaps more important, they dial down the activity of immune foot soldiers that might otherwise cause a massive inflammatory response that would do more damage than the virus itself.
Key players in this two-part bat immune response are interferons, small signaling molecules that got their name because of their talent for interfering with virus replication. They’re a first line of defense for mammals in general: When cells are infected by viruses, they release various interferons as an alarm signal, as do some immune system cells.
But bats seem to go one better. To start with, some species have an outsize number of genes for making interferons. For example, the Egyptian fruit bat (Rousettus aegyptiacus), a natural host of Marburg virus, has 46 such genes (humans have about 20).
Second, species like black flying foxes (Pteropus alecto) keep some genes for making interferons active all the time, even when there’s no viral invader to contend with. One of the things these “always on” interferons do is kick-start production of an enzyme that chops up viral genetic material.
Bats also seem able to tame inflammation, which is essential for fighting infections, but also can be catastrophic.
Out-of-control inflammation is a common theme in severe illnesses from viruses that have jumped from other species: In people infected with deadly filoviruses like Ebola, for example, a bombardment of inflammation-causing molecules spurs the failure of multiple organs and a septic shocklike syndrome.
And some novel strains of influenza, including the one that caused the deadly 1918 pandemic, are especially adept at triggering a barrage of inflammatory molecules. This prolonged blitz — which can manifest as what’s called a cytokine storm — was also found in people who fared poorly with SARS, and is what kills many of the sickest patients with covid-19.
“If you get infected with something, the immune response is always walking this tightrope,” Mandl says. “It’s a balance between making sure the response is vigorous enough — but not so vigorous that, for example, you end up with the lungs filling with fluid and a lot of inflammatory cells.”
Bats walk this tightrope with finesse. They seem to have several ways to avoid the dangerous inflammation overreaction.
For example, a study of immune cells in the greater mouse-eared bat (Myotis myotis) found dialed-up production of interleukin-10, a protein known to suppress the body’s inflammatory responses.
Bats also tamp down activity of large protein clusters called inflammasomes, which coordinate the release of all sorts of inflammation-promoting molecules.
And several bat species no longer make certain proteins that sense damaged genetic material and kick off an inflammatory response. At least 10 species of bats have lost an entire family of such proteins, for example.
Add it all up, and bats “really seem to throttle inflammation,” says evolutionary biologist Emma Teeling of University College Dublin, co-founder of a major effort to explore bat genomes.
As researchers parse how bats coexist with so many viruses, they are also asking why the creatures are this way. The answer may seem surprising: The tolerance could be connected to the fact that bats fly.
Bats are the only mammals capable of sustained, powered flight (they don’t just glide), a feat that required changes in metabolism. Flight is a major workout — estimates suggest a bat’s metabolic rate can increase to up to 34 times over its resting level when it takes to the air. This metabolic uptick during flight generates damaging chemicals called reactive oxygen species that trigger inflammation, which contributes to all sorts of maladies in mammals.
So, scientist hypothesize, by evolving to tamp down flight-associated inflammation, bats may also escape the dangerous inflammation triggered by viral infections.
The study of bats also has more immediate goals. “It’s really important to study bats in nature, to understand where the viruses are so that we can try to understand why they are coming from those populations and killing people,” says epidemiologist David Hayman of Massey University in New Zealand, who wrote an overview on bats and viruses in the Annual Review of Virology.
Recent research suggests that stressful conditions such as food shortages and habitat loss may be key predictors of bats shedding lots of virus. Plowright has spent years capturing bats in giant nets and sampling their blood, urine and feces, and has found that viral infections in bats are not consistent across time and space.
And stress aside, increased contact itself is problematic. “Just the simple fact of more people, more habitat destruction, means more potential contacts, which may just simply increase chances of an infection,” Hayman says.
Trying to eliminate such encounters by eliminating bats is not the answer. “That would be a disaster,” Plowright says. “They provide huge ecosystem services.” Bats are crucial pollinators of hundreds of plants, they aid in seed dispersal, and many are voracious eaters of insects.
Scientists know they’ve only just begun to parse the relationship between bats and the viruses they harbor, or to understand the occasional, catastrophic viral crossovers into our species. With the covid-19 pandemic roiling on, they have expressed dismay and astonishment at the recent termination of funding for some bat and coronavirus research by the National Institutes of Health.
But they are pushing ahead with their work. For his part, Banerjee, now at McMaster University in Hamilton, Ontario, finds himself yet again suited up hazmat-style. He spends his hours nurturing petri dishes of bat kidney cells and growing up flask gardens of virus, then combining the two for infection experiments.
“I thought grad school was busy,” he says. “But this is insanely busy.”
0 notes
Text
Is This The Man Behind The Global Coronavirus Pandemic?

In light of growing speculation, most of it within less than official circles, that the official theory for the spread of the Coronavirus epidemic, namely because someone ate bat soup at a Wuhan seafood and animal market...

... is a fabricated farce, and that the real reason behind the viral spread is because a weaponized version of the coronavirus (one which may have originally been obtained from Canada), was released by Wuhan's Institute of Virology (accidentally or not), a top, level-4 biohazard lab which was studying "the world's most dangerous pathogens", perhaps it would be a good idea for the same Wuhan Institute of Virology to remove the following "help wanted" notice, posted on November 18, 2019, according to which the institute is seeking to hire one or two post-doc fellows, who will use "bats to research the molecular mechanism that allows Ebola and SARS-associated coronaviruses to lie dormant for a long time without causing diseases."
The right candidate will:
Have obtained or is about to obtain a PhD in life science/biomedical related fields;
Have a reliable and rigorous work style, with strong independent scientific research ability and teamwork spirit;
Have strong English communication and writing skills, have research papers published in the international mainstream academic journals
Have a cell biology, immunology, genomics and other relevant background experience is preferred;
The full job posting, which can still be found on the Wuhan Institute of Virology website can be found here (and screengrabbed below as it will be gone within a few hours).

And google translated:
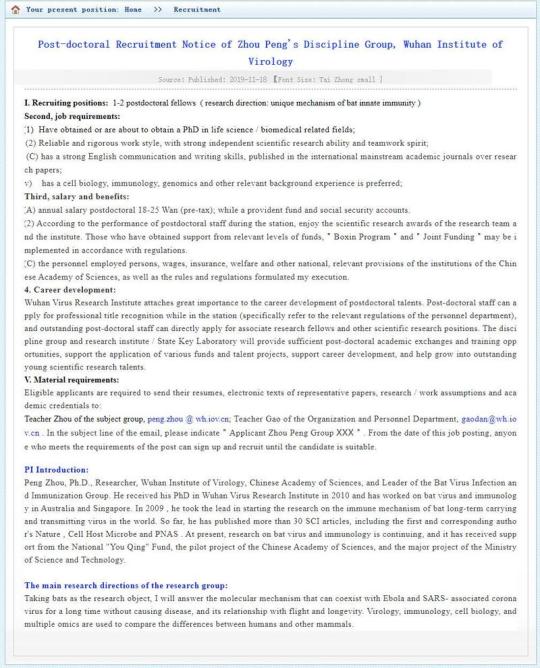
Why is this notable? Because as it turns out, this is a job posting for the lab of Dr. Peng Zhou (周鹏), Ph.D., a researcher at the Wuhan Institute of Virology and Leader of the Bat Virus Infection and Immunization Group. Some more on Zhou's background from the Institute (google translated):
He received his PhD in Wuhan Virus Research Institute in 2010 and has worked on bat virus and immunology in Australia and Singapore. In 2009 , he took the lead in starting the research on the immune mechanism of bat long-term carrying and transmitting virus in the world. So far, he has published more than 30 SCI articles, including the first and corresponding author's Nature , Cell Host Microbe and PNAS . At present, research on bat virus and immunology is continuing, and it has received support from the National "You Qing" Fund, the pilot project of the Chinese Academy of Sciences, and the major project of the Ministry of Science and Technology.
Below is a list of several recent papers published by Dr. Zhou
Dampened STING-Dependent Interferon Activation in Bats
Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin
IFNAR2-dependent gene expression profile induced by IFN-α in Pteropus alecto bat cells and impact of IFNAR2 knockout on virus infection
Immunogenicity of the spike glycoprotein of Bat SARS-like coronavirus
Bat severe acute respiratory syndrome-like coronavirus ORF3b homologues display different interferon antagonist activities
Which brings us to the punchline: courtesy of the Wuhan institute of virology, here is a press release from Dr. Zhou's lab titled "How bats carry viruses without getting sick":
Bats are known to harbor highly pathogenic viruses like Ebola, Marburg, Hendra, Nipah, and SARS-CoV, and yet they do not show clinical signs of disease. In a paper published in the journal Cell Host & Microbe on February 22, scientists at the Wuhan Institute of Virology in China find that in bats, an antiviral immune pathway called the STING-interferon pathway is dampened, and bats can maintain just enough defense against illness without triggering a heightened immune reaction.
"We believe there is a balance between bats and the pathogens they carry," says senior author Peng Zhou. "This work demonstrated that in order to maintain a balance with viruses, bats may have evolved to dampen certain pathways."
In humans and other mammals, an immune-based over-response to one of these and other pathogenic viruses can trigger severe illness. For example, in humans, an activated STING pathway is linked with severe autoimmune diseases.
"In human history, we have been chasing infectious diseases one after another," says Zhou, "but bats appear to be a 'super-mammal' to these deadly viruses." By identifying a weakened but not defunct STING pathway, researchers have some new insight into how bats fine-tune antiviral defenses to balance an effective, but not an overt, response against viruses.
The authors hypothesize that this defense strategy evolved as part of three interconnected features of bat biology: they are flying mammals, have a long lifespan, and host a large viral reservoir.
"Adaptation to flight likely caused positive selection of multiple bat innate immune and DNA damage repair genes," Zhou says. These adaptations may have shaped certain antiviral pathways (STING, interferon, and others) to make them good viral reservoir hosts and achieve a tolerable balance."
And just in case, here is a google-translated press release from Jan 18, 2019 describing the achievements of Dr. Peng Zhou:
Wuhan has the first person in the global bat immunity research: "I rushed forward with a sword"
Changjiang Daily Financial Media May 4 hearing last month as they tied for first author made a "natural", in recent years, the Chinese Academy of Sciences Wuhan virus after 80 young researchers Zhou Peng has been in the "natural", "American Academy of Sciences ”And other international authoritative magazines published 28 papers, becoming academic stars. In an interview with reporters recently, he introduced that young scientists do not rely on genius to hold, but rely on "super confident".
It is understood that Zhou Peng is the pioneer of global bat immune system research. "Bats carry viruses but do not get sick. They have not been researched by scientists before, and certainly have specificity different from other species, but this is like you know the beginning and Ending without knowing how the story happened. " After more than 10 years of research, Zhou Peng discovered that an antiviral immune channel called "interferon gene-stimulating protein-interferon" in the bat's body was inhibited, so that the bat could just resist the disease without triggering a strong immune response. The results were published in Cells, Hosts and Microorganisms, which aroused the attention of the academic community.
Zhou Peng, a student of undergraduate bioengineering, experienced SARS (Severe Acute Respiratory Syndrome) in his junior year, which made him interested in the virus: "A small virus makes the world mess." He was admitted to the Wuhan Institute of Virology of the Chinese Academy of Sciences at the postgraduate level, and studied under Shi Zhengli, a bat expert. Focusing on the virus carried by the bat, then I was wondering if the bat's immune system is special. "
After graduating from the PhD, he entered the Australian Animal Health Laboratory and became the first person in the global bat immunity research. "I went through 4 years of trial and error, groped in the dark, and hit the South Wall numerous times. I still remember a 'darkest moment' 'In the local cold winter, I was holding the frostbite knee, sitting at the beach, and asking myself why this was the case.'
He began to learn Australian jokes and inspired himself. In 2016, during postdoctoral studies at Duke University-National University of Singapore Medical School, he was concerned that a certain interferon in bats is always maintained at a high level. This paper became the cover article of the Proceedings of the National Academy of Sciences, "Bat Immunity "This door was opened, and more and more people in the world are paying attention to this field." Our generation, when we were in college, watched "The Forrest Gump" and "Redemption of Shawshank" and taught us stupidity and perseverance. I I feel like I am carrying a sword and rushing forward. "
After returning to China in 2016, Zhou Peng returned to his alma mater to become a little-known young researcher. "In the long run, bats carry the virus without getting sick. It is hoped that humans can learn how to fight the virus, but this is still far from industrialization. Far, the road ahead is long, and we must remain 'super confident' and continue to move forward. "(Reporter Li Jia correspondent Chen teased Li Li intern Luo Yameng)
His bio (source):
Peng Zhou, Ph.D., researcher, team leader of bat virus infection and immunity. He successively obtained bachelor's and doctoral degrees from Henan University (2004) and Wuhan Institute of Virology, Chinese Academy of Sciences (2010). During his doctorate, he was sent to the Australian Animal Health Laboratory for study. He then carried out research work at Duke-Nus Medical College in Australia and Singapore. He has long been engaged in the research of new virus epidemiology and bat antiviral immunity, revealing that bats carry SARS, MERS, and Ebola for a long time but do not have their own immune mechanisms.
Currently he is hosting and undertaking 3 projects of the National Natural Science Foundation of China, and the Chinese Academy of Sciences Special project and a major national science and technology project - a major project for the prevention and control of infectious diseases. Currently published 28 SCI papers, including Nature, Cell Host Microbe, PNAS and other articles SCI papers, including Nature, Cell Host Microbe, PNAS and other articles published by the first or corresponding author. It is at the forefront of the world in the field of bat and virus research.
So to summarize:
One of China's top virology and immunology experts was and still works at China's top-rated biohazard lab, the Wuhan Institute of Virology, which some have affectionately called the real Umbrella Corp.
Since 2009, Peng has been the leading Chinese scientist researching the immune mechanism of bats carrying and transmitting lethal viruses in the world.
His primary field of study is researching how and why bats can be infected with some of the most nightmarish viruses in the world including Ebola, SARS and Coronavirus, and not get sick.
He was genetically engineering various immune pathways (such as the STING pathway in bats) to make the bats more or less susceptible to infection, in the process potentially creating a highly resistant mutant superbug.
As part of his studies, Peng also researched mutant Coronavirus strains that overcame the natural immunity of some bats; these are "superbug" Coronavirus strains, which are not resistant to any natural immune pathway, and now appear to be out in the wild.
As of mid-November, his lab was actively hiring inexperienced post-docs to help conduct his research into super-Coronaviruses and bat infections.
Peng's work on virology and bat immunology has received support from the National "You Qing" Fund, the pilot project of the Chinese Academy of Sciences, and the major project of the Ministry of Science and Technology.
* * *
Something tells us, if anyone wants to find out what really caused the coronavirus pandemic that has infected thousands of people in China and around the globe, they should probably pay Dr. Peng a visit.
https://www.zerohedge.com/health/man-behind-global-coronavirus-pandemic
0 notes
Text

Young black flying fox bat
The black flying fox or black fruit bat (Pteropus alecto) is a bat in the family Pteropodidae. It is among the largest bats in the world, but is considerably smaller than the largest species in its genus, Pteropus. The black flying fox is native to Australia, Papua New Guinea, and Indonesia. It is not a threatened species.
📷 Ken Drake
71 notes
·
View notes
Photo
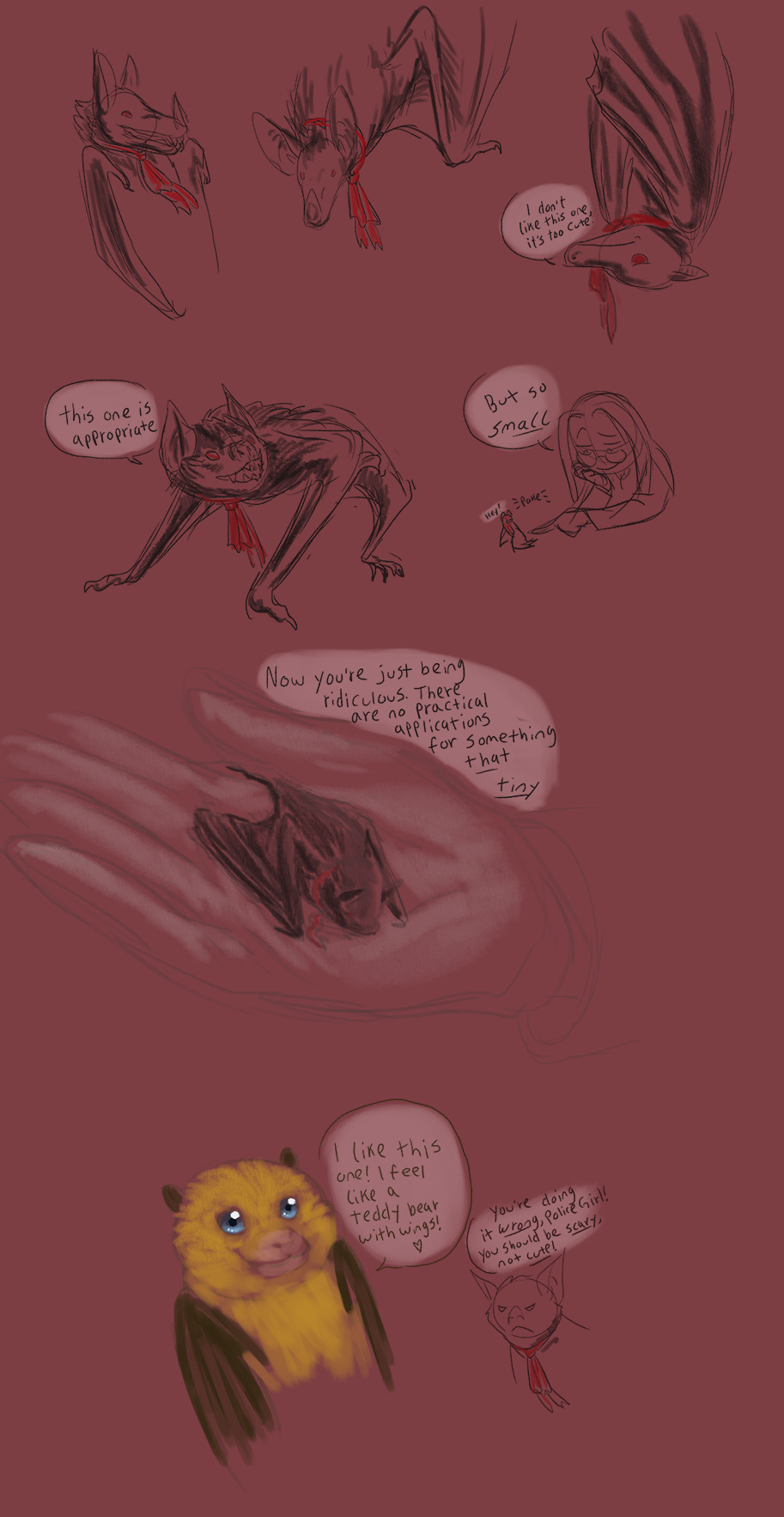
Not doggos but still Hellsing (there will be more doggos, I promise.)
I was about to go watch tv when a discord buddy mentioned Spectral Bats in a Hellsing context and that just threw all my plans out the window because three things I love to death are:
Bats
Vampires
Drawing characters as (specific) animals
And so here you have a bunch of Alubats. Enjoy.
For all the other nerds like me, the species list is under the cut.
Spectral Bat (Vampyrum spectrum) top row, left and center.
Black Flying Fox (Pteropus alecto) top row, right.
Common Vampire Bat (Desmodus rotundus) entire second row.
Kitti’s Hog-nosed Bat aka Bumblebee Bat aka Smallest Living Mammal (Craseonycteris thonglongyai) third “row.”
Cameo of SerasBat as a Fijian Monkey-faced Bat (Mirimiri acrodonta) bottom, with disappointed BatDad as a Common Vampire again.
#hello my name is comix and I am bat trash#even before I was bird trash#art#doodles#hellsing#bats#alucard#Seras Victoria#Integra Fairbrook Wingates Hellsing#hellsing ultimate
343 notes
·
View notes
Text



ZORROS VOLADORES
(Pteropus)
Género de murciélagos frugívoros conocidos comúnmente como zorros voladores
Localización:
Habitan en las regiones tropicales de África, Asia, y otras islas de Oceanía; principalmente Australia y Filipinas
Características:
A diferencia del resto de sus parientes, ven bien y no pueden volar en la oscuridad
Su sistema de localización mediante eco está poco desarrollado
Son principalmente comedores de fruta y diseminan las semillas de muchas plantas
Ocasionalmente duermen en grandes colonias, colgados de los árboles
Especies:
Pteropus Admiralitatum
Pteropus Aldabrensis
Pteropus Alecto
Pteropus Anetianus
Pteropus Aruensis
Pteropus Brunneus †
Pteropus Caniceps
Pteropus Capistratus
Pteropus Chrysoproctus
Pteropus Cognatus
Pteropus Conspicillatus
Pteropus Dasymallus
Pteropus Faunulus
Pteropus Fundatus
Pteropus Giganteus
Pteropus Gilliardorum
Pteropus Griseus
Pteropus Howensis
Pteropus Hypomelanus
Pteropus Insularis
Pteropus Intermedius
Pteropus Keyensis
Pteropus Leucopterus
Pteropus Livingstonii
Pteropus Lombocensis
Pteropus Loochoensis
Pteropus Lylei
Pteropus Macrotis
Pteropus Mahaganus
Pteropus Mariannus
Pteropus Melanopogon
Pteropus Melanotus
Pteropus Molossinus
Pteropus Neohibernicus
Pteropus Nitendiensis
Pteropus Ocularis
Pteropus Ornatus
Pteropus Pelewensis
Pteropus Personatus
Pteropus Pilosus †
Pteropus Pohlei
Pteropus Poliocephalus
Pteropus Pselaphon
Pteropus Pumilus
Pteropus Rayneri
Pteropus Rennelli
Pteropus Rodricensis
Pteropus Rufus
Pteropus Samoensis
Pteropus Scapulatus
Pteropus Seychellensis
Pteropus Speciosus
Pteropus Subniger †
Pteropus Temminckii
Pteropus Tokudae †
Pteropus Tonganus
Pteropus Tuberculatus
Pteropus Ualanus
Pteropus Vampyrus
Pteropus Vetulus
Pteropus Voeltzkowi
Pteropus Woodfordi
Pteropus Yapensis
0 notes
Photo

The black flying fox (Pteropus alecto) will carry her young with her when she flies out in search of food for the first month of its life. via /r/Awwducational http://ift.tt/2rgeIde: http://ift.tt/2mLsiRf
1 note
·
View note