#MeCN
Note
yoooo rodney sala mecn ???!@?!???
hell yeah man that’s me
2 notes
·
View notes
Text
Cleavage Reagent for DNA Synthesis
Cleavage Reagent for DNA Synthesis
Catalog number: B2012914
Lot number: Batch Dependent
Expiration Date: Batch dependent
Amount: 50 mL
Molecular Weight or Concentration: N/A
Supplied as: Solution
Applications: molecular tool for various biochemical applications
Storage: 2-8°C
Keywords: 20% DEA in MeCN (v/v)
Grade: Biotechnology grade. All products are highly pure. All solutions are made with Type…

View On WordPress
0 notes
Text
Liquid as well as pharmacological real estate agents for bond elimination right after gynaecological surgical treatment
Inside the CA2/3 region, cyclin D1 immunoreactivity had been above that will within the CA1 area and never altered soon after ischemia/reperfusion. Inside the dentate gyrus, chronological change in cyclin D1 immunoreactivity ended up being observed. Cellular material in the granule cell level showed unique alteration of cyclin D1 immunoreactivity after ischemia/reperfusion: the cyclin D1 immunoreactivity has been cheapest with Twelve hrs and robust One along with 4 times following ischemia/reperfusion. In addition, alternation in cyclin D1 proteins degree is discovered within the ischemic hippocampus. Conclusion: Our benefits show which cyclin D1 may participate in a huge role inside cell phone occasions related to neuronal damage subsequent ischemia/reperfusion.Pyrimidine nucleoside phosphorylase through Bacillus subtilis (BsPyNP, Electronic.Chemical. Two.Some.A couple of.3) along with thymidine phosphorylase via Escherichia coli (EcTP, Elizabeth.C. Two.Several.A couple of.Several) were chosen, because incapacitated enzymes, within the activity associated with 5-halogenated pyrimidine 2'-deoxyribonucleosides (14-18) by simply transglycosylation throughout completely aqueous method. In the marketplace analysis review of the two biocatalysts, simply no exceptional differences appeared regarding their substrate specificity, bioconversion produce, balance inside natural and organic cosolvents (DMF as well as MeCN). In addition, each biocatalysts could be remade not less than 5 times without decrease of the actual efficiency. Both enzymes tend not to ABT-267 take arabinonucleosides along with 2',3'-dideoxynucleosides while substrates, although they will catalyze bioconversions regarding 5'-deoxyribonucleosides along with 5-halogenated uracils. The actual combination of compounds 14-18 they proceeded in a equivalent transformation (33-68% regarding BsPyNP as well as 25-62% regarding EcTP, respectively). Immobilization is discovered to be able to put in, for the biocatalysts, an impressive enhancement of stableness after incubation throughout MeCN. Optimization involving 5-fluoro-2'-deoxyuridine (14) synthesis (ph 6.5, 10 millimeter phosphate stream, nucleoside/nucleobase Three or more:1 molar percentage) as well as future scale-up provided the target chemical substance within 73% (EcTP) as well as 76% (BsPyNP) transformation (about Nine g/L). (chemical) 2013 Elsevier W./. Most privileges set aside.Target: Countrywide dosimetry audits can be a basic a part of quality confidence within radiotherapy, specifically for brand new tactics. Intraoperative radiotherapy having a lightweight portable kilovoltage X-ray origin is often a fresh approach for treating breast and also other malignancies. Most several current medical sites in the UK ended up audited by way of a one visiting party and hang of dimension tools. Methods: Sizes associated with end result, isotropy along with degree amounts ended up executed having an chamber within sound drinking water, thermoluminescent dosemeters and radiochromic film, respectively. Results: The actual suggest difference between tested and also prepared dosage around most revolves was -3.2 +/- Only two.7%. Tested isotropy had been inside of +/- 3% around the side airplane of the X-ray supply as well as +11 +/- 4% from the onward direction in comparison with the particular lateral plane. Assessed detail doses were agreed inside of 5 +/- 2% associated with manufacturer-provided calibration ideals or a indicate gamma list of 97% with a building up a tolerance regarding 7%/0.5mm. Conclusion: Deal inside way of measuring questions was found for many about three details except forwards anisotropy, which can be improbable being clinically considerable.
#PI3K Inhibitor Library#JNK inhibitor#PD-1/PD-L1 inhibitor#IU1#Shikonin#Paeoniflorin#Zebularine#Pitavastatin#Caspofungin#Fisogatinib#SANT-1#Piperlongumine#Pioglitazone#Go6976#Asunaprevir#StemRegenin 1#AZD7545#Mifepristone#EED226#Adavivint#NSC-9900#R 41400#ZK-62711#MK-1439#GS 0840#BMS-232632#GS5885#VX-478#ABT-267#ABT-450
1 note
·
View note
Text
7440-31-5 Synthesis and structural characterization of carbons-adjacent stannacarboranes of the C<sub>2</sub>B<sub>10</sub> system
The chemistry of p-block metallacarboranes of the C<sub>2</sub>B<sub>10</sub> systems is largely unexplored in comparison with that of s-, d-, and f-block metallacarboranes. This article reports several carbons-adjacent stannacarboranes of the C<sub>2</sub>B<sub>10</sub> system and their chemical properties for the first time. Reaction of SnCl<sub>2</sub> with [{μ-1,2-[o-C<sub>6</sub>H<sub>4</sub>-(CH<sub>2</sub>)<sub>2</sub>]-1,2- C<sub>2</sub>B<sub>10</sub>H<sub>10</sub>}<sub>2</sub>Na<sub>4</sub> (THF)<sub>6</sub>]<sub>n</sub> gave the Lewis base free stannacarborane {μ-1,2-[o-C<sub>6</sub>H<sub>4</sub>(CH<sub>2</sub>)<sub>2</sub>]-1,2- C<sub>2</sub>B<sub>10</sub>H<sub>10</sub>}-Sn (1). Recrystallization of 1 from MeCN, THF, and DME afforded the corresponding Lewis base coordinated stannacarboranes {μ-1,2-[o-C<sub>6</sub>H<sub>4</sub> (CH<sub>2</sub>)<sub>2</sub>]-1,2-C<sub>2</sub>B<sub>10</sub>H<sub>10</sub>} Sn(MeCN) (2), {μ-1,2-[o-C<sub>6</sub>H<sub>4</sub> (CH<sub>2</sub>)<sub>2</sub>]-1,2-C<sub>2</sub>B<sub>10</sub>H<sub>10</sub>}Sn (THF)·THF (3·THF), and {μ-1,2-[o-C<sub>6</sub>H<sub>4</sub> (CH<sub>2</sub>)<sub>2</sub>]-1,2-C<sub>2</sub>B<sub>10</sub>H<sub>10</sub>}Sn (DME) (4), respectively. They were fully characterized by various spectroscopic data and elemental analyses. Complexes 2-4 were further confirmed by single-crystal X-ray analyses.
0 notes
Text








voca meme's from shit me and my friends have said
#vocaloid#sf a2 miki#iroha nekomura#hime meika#xin hua#kizuna akari#yuzuki yukari#iori yuzuru#ia vocaloid#moke zhiyu#fukase vocaloid#piko utatan#kyo vocaloid#gumi megpoid#macne nana#macne petit#mecne coco black#luka megurine#hatsune miku
51 notes
·
View notes
Text
SEVGİLİYİ İNCİTİRSİN (Mesnevi'den)
Mecnûn, ayrılık derdinden aniden rahatsızlandı. Boğazı tıkandı. Tedavi için doktor çağırdılar. Mecnûn'u muayene eden doktor, ''Pis kanı almak için hacamat olması gerekir'' dedi.
Hemen bir hacamatçı çağırdılar. Hacamatçı kanını almak için,Mecnûn'un kolunu bağladı. Neşterle tam kesecekken Mecnûn bir nâra atarak,
''Paranı al git. Kan almayı bırak. Ölürsem bu dertten öleyim''
dedi. Hacamatçı,
''Kükremiş aslandan bile korkmazken, bundan niye korktun?Geceleri bütün vahşi hayvanların etrafında toplandığını biliyorum'' dedi. Mecnûn,
''Ben senin açacağın yaradan korkmam. Sabrım, tahammülüm dağlardan fazladır. Fakat bütün bedenim Leylâ ile dolu olduğu için, ona bir zarar gelmesinden korkarım. Gönlü uyanık olan kişiler bilir ki, Leylâ ile benim aramda fark yoktur'' diyerek kanını aldırmadı.
***
Varlığımda bir addan başka bir şey kalmadı. Ey güzelim,vücudumda senden başka varlık yok. Bu sebeple sirke ve bal denizde nasıl yok olursa, ben de sende öyle yok oldum.
27 notes
·
View notes
Text
Global Acetonitrile (ACN, CAS 75-05-8) Market – Sales and Forecast to 2027
Buy Now
Acetonitrile, also well-known as cyanomethane or abbreviated MeCN, is the chemical compound with the formula CH3CN. It is utilized in organic synthesis, acrylic fibers, pharmaceuticals, perfumes, and innitrile rubber. Acetonitrile is also utilized as a solvent and as a chemical intermediate.
According to the report analysis, ‘Global Acetonitrile (ACN, CAS 75-05-8) Market, 2021-2027’ states that AnQore B.V., Asahi Kasei Corporation, Balaji Amines Ltd., Changyi Ruihai Biotechnology Co., Ltd., Chemax International Corporation, China National Petroleum Corporation, China Petroleum & Chemical Corporation (Sinopec), Formosa Plastics Corporation, INEOS Group Limited, Maharashtra Aldehydes & Chemicals Ltd. (MACL), Mitsubishi Chemical Corporation, Nantong Acetic Acid Chemical Co., Ltd., Nova Molecular Technologies, Inc., Petrleos Mexicanos, PJSC Lukoil Oil Company, Rhythm Chemicals Pvt. Ltd., Shandong Huihai Pharmaceutical & Chemical Co., Ltd., Shanghai SECCO Petrochemical Co., Ltd. (SECCO), Taekwang Industrial Co., Ltd., Tongsuh Petrochemical Corp., Ltd., among others are the chief market players which presently operating in the global acetonitrile (ACN, CAS 75-05-8) market more proficiently for keep maintaining the governing position, leading the highest market growth, registering the great value of market share, generating the highest percentage of revenue, and obtaining the competitive edge by establishing the several research and development programs, analysing the strategies and policies of government as well as similar entities, improving the qualitative and quantitative measures of such, implementing the policies of profit making and strategies of expansion, spreading the awareness connected to the applications and advantages of acetonitrile, increasing the benefits and features of acetonitrile and analysing the strategies and policies of government as well as similar entities.
On the basis of product, the ‘Global Acetonitrile Market’ is classified into technical grade acetonitrile and high purity acetonitrile. On the basis of application, the global acetonitrile market is classified into chemical intermediates, fibers, lithium batteries, petrochemicals, pharmaceuticals, plastics and textile.
Rising demand for acetonitrile in various industries such as pharmaceuticals and specialty chemicals has emerged as the key growth driver for the global acetonitrile market. Acetonitrile is also extensively used as a solvent in the manufacturing of insulin and other antibiotics.
In addition to this, surge in acetonitrile requirement in the production of acrylic fibers and plastics is also supporting the growth of the complete acetonitrile market. The agricultural industry has observed a shift in preferences during recent times, with aqueous acetonitrile observing a surge in demand. The growing deployment of aqueous acetonitrile for agricultural activities is predicted to have a positive influence on the complete acetonitrile market. Acetonitrile has also observed an augmented demand in other procedures such as drug recrystallization, which is predicted to propel the growth in the acetonitrile market, during the forthcoming years.
Request for Sample Report @ https://www.kenresearch.com/sample-report.php?Frmdetails=NDkzMTE4
Furthermore, leading players could utilize the acetonitrile as a derivative to produce new products, which would deliver the more benefits than predecessor products and could observe increased requirement, thereby assisting in the growth of acetonitrile market. R&D undertakings could be a foremost growth strategy for acetonitrile market players, during the coming years as well. Therefore, it is predicted that during the near years the market of acetonitrile will increase around the globe more progressively over the near future.
For More Information, refer to below link:-
Global Acetonitrile (ACN, CAS 75-05-8) Market Research Report, 2021-2027
Related Reports
Global 1,2-Dichloroethane (EDC, CAS 107-06-2) Market, 2021-2027
Global Acetonitrile (ACN, CAS 75-05-8) Market Outlook 2018-2023
Follow Us
LinkedIn | Facebook | Twitter | YouTube
Contact Us:-
Ken Research
Ankur Gupta, Head Marketing & Communications
+91-9015378249
#Global Acetonitrile Market#Global Acetonitrile Industry#Global Acetonitrile Market Revenue#Global Acetonitrile Market Share#Global Acetonitrile Market Size#Global Acetonitrile Market Growth#Global Acetonitrile Market Trends#Global Acetonitrile Market Demand#Global Acetonitrile Market Competition#Global Acetonitrile Market Analysis#Industry Research Report Of Global#Global Acetonitrile Industry Research Report#Global Acetonitrile Research Report#Acetonitrile Market In Global
0 notes
Text
The Web Based Variation of Dominobet
From Chinese emperor era, played as domino card game in 10th century to the latest 17th century played as ‘poque’in France Poker carries a large record. But it gained popularity between troops in the American Civil Warfare, and eventually migrated on the European frontier. Domino bet has gained substantial pursuing in recent times due to involvement of mass media. Internet has made the accessibility game really easy that any person may play it without going to casino or gambling place. In India betting remains to be illegal in most of says but individuals circumvent the laws as there is usage of simple and suitable online. Internationally domino bet is simply a game of opportunity, good fortune and talent however in India any game that involves chance is against the law, solely those game titles are thought legal which involve ability and work.
The status:
Based on MECN (Mass media Entertainment Consulting Group) India provides the swiftest developing readers as everyone is now agreeing to it a expertise-based game, because of in which the far more it rolls the better it expands as being a snowball. Now there is absolutely no converting rear both we acknowledge it or not domino bet has beginning to understand its lower leg from the Native Indian gaming market and sooner or later it would seize the main share on the market right now.

Online domino bet has only managed to make it easy to entry the enjoyment of domino wager to a beginner individual without having the problem to likely to players or gambling establishment, all they need is really a system and a confirmed app and a suitable internet connection. Nowadays, different websites on the internet domino bet India offer you a variety of tournaments throughout the full week that consistently entice developing phone numbers. But in addition internet gambling is legal in this article as only when it is applied like a purpose of pleasure along with the sites are deducing TDS from earnings. There will be variations in the law for casino once things resolve down.
The range of playing the game right now!
Initially domino bet in India was really a masculine dominated game which designed ladies had been not dealt with just as on domino bet kitchen table. The good news is as organizations and web sites ought to gain more profit they welcome females to try out far more, they are produced comfy. Domino bet is lucrative past time game for most females in today’s time.
Retaining all the profit, enjoyable and pleasure to one side, domino bet even features a darkish area that contains of tension, drugs along with the whole felony act experiencing these internet websites. Several domino bet players forfeit their families and partnerships, they start alienating individuals who are not coupled to the game click here. Or else appropriately licensed and legalized domino bet will surely use a unfavorable effect on the community.
0 notes
Text
عدم تطابق المرحلة المخففة والمحمولة الخاصة بك «ChromaBLOGraphy

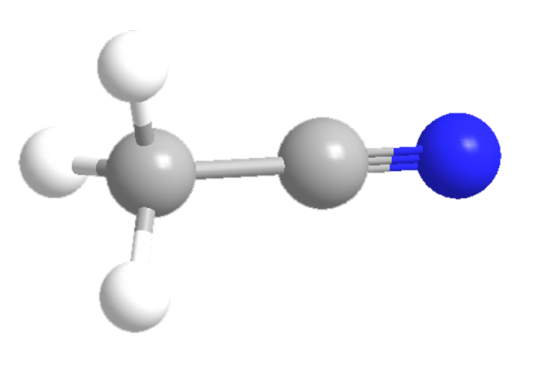
إن الدرس المبكر الذي يتعلمه معظمنا في التحليل الكروماتوجرافي السائل هو: قم بمطابقة مخففك دائمًا بمرحلة هاتفك المحمول. بمجرد الانتهاء من الامتحانات ، إذا تعلمت ذلك في إحدى دورات الكلية ، أو إذا ابتعد مديرك عن العمل ، فإنك تبدأ في استعراض هذا "الشرط" قليلاً لمعرفة مدى التطابق الذي يحتاجون إليه. فهمتها! لا تريد أن تضطر إلى التخلص من جميع المواد العضوية ، لأن ذلك يستغرق بعض الوقت. أو إذا كنت تكتشف عدة أعمدة ، فأنت تريد فقط استخدام نفس العينة التي استخدمتها في اختبارات HILIC واختبارات الطور العكسي. في بعض الحالات ، يمكنك أن تفلت من هذا ربما لأن حليلتك لديها الكثير من التقارب لمرحلة قرطاسية الثابتة الخاصة بك ، وهذا الجزء الإضافي من المذيبات القوية لا يكفي لتدمير انفصالك. بينما في حالات أخرى ، تجاوزت تحليلاتك بشكل جيد الحجم الميت للعمود ، لذلك لا يزال شكل الذروة جيدًا. في هذه الحالات ، يمكن أن يكون موافقًا لثني "القواعد" قليلاً. رغم ذلك ، في بعض الأحيان ، لديك مادة تحليلية حساسة للغاية ، بحيث أن الأسيتونيتريل المتبقي بنسبة 5٪ في مخففك يكفي لتدمير اللوني تمامًا. أقدم لكم دراسة الحالة هذه على مادة الأكريلاميد. الأكريلاميد هو جزيء قطبي صغير (إظهار الصورة). هو أقرب إلى مسحوق Iocane. إنه عديم الرائحة ، لا طعم له ، يذوب على الفور في السائل ، ولا يعد من بين السموم القاتلة التي يعرفها الإنسان. رغم أنه كملاحظة جانبية ، يُعتقد أنه مادة مسرطنة محتملة التعرض المتكرر لفترة طويلة. في الواقع ، الأكريلاميد قابل للذوبان في الماء والميثانول وقابل للذوبان في الأسيتونيتريل. بالإضافة إلى ذلك ، يصعب الاحتفاظ بها على معظم أعمدة LC. في وقت سابق من هذا العام ، قمنا بتطوير منتج خصيصًا لتحليل الأكريلاميد وقمنا بتطوير تطبيقات في مختلف المصفوفات. في وقت مبكر من تطوير الطريقة ، لاحظنا أن مادة الأكريلاميد كانت حساسة للغاية لبقايا المذيبات العضوية المتبقية من إجراء تحضير العينة. في طريقة EN 16618 المنشورة ، يكون المستخلص النهائي في شح الماء / الميثانول. الخطوة قبل التحليل هي ضربة طويلة لأسفل. خطوة كثيرة ، بما في ذلك أنا ، يغري لتغيير قصير. في طريقة QuEChERS ، يكون مخفف العينة النهائي هو الأسيتونيتريل (MeCN) ، وهو أكثر ضررًا بالتحليل الكروماتوجرافي ويتطلب استبدال المذيبات بالكامل. لفهم حساسية الأكريلاميد بشكل أفضل تجاه المادة المخففة العضوية ، أجرينا عمداً عملية استخراج الأكريلاميد من ��قائق البطاطس / رقائق البطاطس وأضفنا مذيبًا عضويًا بأحجام معروفة لنرى كيف غير ذلك الكروماتوغرافيا من عينة الإعدادية المثالية إلى عينة قد تكون أكثر "كسولة" في طريقتنا ، نستخدم الطور المتحرك المائي بنسبة 100٪ مع حمض الفورميك بنسبة 0.001٪ ، مما يعني أن المخفف يجب أن يكون مائياً بنسبة 100٪. نظرًا لأنه تمت إضافة المزيد من المواد العضوية إلى المادة المخففة ، انخفض وقت الاستبقاء واتسع عرض الذروة. في حين أن 10 ٪ من الميثانول في المادة المخففة لا يزال يعطي الاحتفاظ اللوني والكروماتوجرافي ، أدى 20 ٪ أو أكثر من الميثانول إلى سوء شكل الذروة والاحتفاظ بها. في حالة وجود MeCN المتبقي حتى 5٪ ، حدث توسع هائل في الذروة وعندما حدث المزيد من MeCN ، انقسم ذروة الأكريلاميد ثم تلاشى في النهاية مع ذروة ملوث المصفوفة. على الرغم من أن مادة الأكريلاميد مؤهلة كمثال صارخ ، إلا أن هذه البيانات تُظهر ضرورة التأكد من أن مادة المخفّف لديك تتطابق مع مرحلة بدء تشغيل هاتفك المحمول. لاحظنا أيضًا أن وجود عضوي قوي في روتين الشطف التلقائي الخاص بك قد يؤدي إلى تأثير مماثل على اللوني الخاص بك. لذلك في عام 2020 ، وبعده ، في المرة القادمة التي تشاهد فيها تحليل كروماتوجرافي رديء مع ذروة مبكرة أو قمم ضعيفة الاحتفاظ بها ، ضع في اعتبارك التحقق من تركيبتك المخففة. قد يكون حلك بسيطًا مثل التأكد من أن مادة المخفف تتوافق بشكل أفضل مع مرحلة هاتفك المحمول. سنة جديدة سعيدة منا جميعًا هنا في رستك! تم نشر هذا الإدخال في يوم الثلاثاء ، 31 كانون الأول (ديسمبر) 2019 ، الساعة 9:14 مساءً ، ويودع تحت Enviro، Food، LC، LC-MS / MS، Tips & Tricks، Troubleshooting. يمكنك متابعة أي ردود على هذا الإدخال من خلال موجز RSS 2.0. يمكنك تخطي إلى النهاية وترك الرد. والأزيز حاليا لا يسمح.
0 notes
Text
Promoted by Samarium Reaction of (3r,4r)-3-((lr)-l-{[Tert-Butyl(dimethyl)Silyl]oxy}Ethyl)-4-Acetoxy-Azetidin-2-one with Methyl 2-Bromopropianate. Unusual Decyclization of Azetidin-2-one Derivative in Approaches to Carbapenems Analogues-JuniperPublishers
To know more about Journal of chemistry,
Click here: https://juniperpublishers.com/omcij/index.php
To know more abour juniper Publishers,
click here: https://juniperpublishers.com/index.php

Abstract
The reaction of the title compound 1 with Sm-reagent prepared from powdered Sm, catalytic amounts of I2 and methyl 2-bromopropionate in THF, leads to the anomalous substituted product 3. The alkylation of the last compound with methyl bromoacetate gives methyl 2-[(2S,3S)- 3-((1R)-1-{[tert-butyl(dimethyl) silyl]oxy}ethyl)-1-(2-methoxy-2-oxoethyl)-4-oxoazetidine-2-yl]-2-methyl-3-oxopentanoate 5 which under the action of NaHMDS in THF at -78° undergoes fragmentation with a disconnection of N1-C4-bond and the formation of acyclic amide 7. Possible stepwise formation routes of 3 and 7 are discussed.
Introduction
Antibiotics of β-lactam series are one of the most popular drugs against infectious diseases. However, microorganisms quickly produce resistance against the used drugs. Now this problem is not solved, but the time of production of this resistance can be increased by introducing new compounds into practice or by modifying the known ones [1,2]. The key block 2 used in the synthesis of practically important 1β-methylcarbapenems (meropenem, ertapenem, doripenem and etc) has been obtained by alkylating azetidinone 1 with Zn-, Li-, B-, Sn- enolates of propionic acid derivatives (amides, thioethers, thia- and oxazolidones, etc.) [3].
We did not find any literature data on the reaction of 1 with Sm-enolates of propionic acid esters. In this paper, in order to obtain new structures, we studied the Sm-promoted Reformat sky Reaction 1 and methyl 2-bromopropianate was carried reaction of azetidin-2-one 1 [4] with methyl 2-bromopropionate.
Results and Discussion
Reaction 1 and methyl 2-bromopropianate was carried reaction of azetidin-2-one 1 [4] with methyl 2-bromopropionate. out in a THF using metallic samarium powder and catalytic amounts of 12 [5]. After the initial azetidinone 1 was consumed, the reaction mass was quenched with aq. NH4Cl. The major alkylation product 3 was isolated in a 70% yield as a 2:1 mixture of diastereoisomers differing by the configurations of the side chain center. The side product azetidinone 4 [6] was isolated in a 5% yield. The subsequent use of 3 was planned according to the traditional methodology for the synthesis of carbapenems from 1 [7-9] through N-alkylation steps of 3 with methyl bromoacetate followed by intramolecular Dieckmann cyclization of adduct 5 to produce precursor 6 (Scheme 1).
As expected, the alkylation step of 3 with methyl bromoacetate proceeded smoothly with a good yield, leading to 5 as the inseparable (SiO2) mixture of diastereomers in a ratio of 2: 1. An attempted intramolecular cyclization of 5 (NaHMDS,THF,-78°C) failed to produce 6. Instead, a rapid formation of a 1:1 diastereomeric mixture of acyclic amides 7 was observed (Scheme 2).
Thus, there was an unusual decyclization of azetidinone 5 at the N1-C4 bond occurring in the reaction. To the best of our knowledge, there were no precedents described for 5 decyclizations in literature. The possible mechanisms of 3 and 7 formation are also of synthetic interest. Obviously, the generation of the Sm reagent from methyl 2-bromopropianate in the synthesis of 3 was preceded by the Claisen-type condensation of two molecules of the bromoester with the formation of β-ketoester 8 (Scheme 3). The generation of enolate 9 with the removal of Br led to stable enolate 10. The latter smoothly reacted with inline 11 formed under the experimental conditions from 1 to give 3.
Numerous examples of the SmI2-promoted Reformatsky type of inter- and intramolecular reactions of α-halo ketones and α-halo-ethers [10-14] have been described in the literature. Thus, the reactions of ethyl α-bromoacetate and α-bromopropionate with Sml2 proceed with the generation of expected Sm reagents that are trapped with carbonyl compounds or isolated as selfcondensation products (β-keto esters)[15-18]. In our case, the nature of the Sm-reagent is slightly different, because instead of Sml2 we used metallic samarium and catalytic amounts of l2 according to the method of the Banik and Basu [5]. All this influence on the results of the reaction, for example, the formation of 4 the product of the reduction of the intermediate imine 11.
A possible mechanism of 7 generation is presented in Scheme 4. The transient carbanion 12 generated from 5 with NaHMDS can be fragmented in directions a or b. The classical variant (a) with the release of the methoxide anion and the formation of the ketone 6 was not realized and 12 undergoes disintegration by path b, leading after aqueous treatment of the reaction mixture to the acyclic amide 7 (Scheme 4). The driving force for fragmentation 12 by path b is the removal of steric hindrance and the formation of a thermodynamically advantageous enone system 7. Protonation in the acyclic structure A is not stereoselectively and from the initial 2:1 mixture of diastereomers 5, a 1:1 mixture of diasteroisomers of amide 7 was obtained. Any possible isomers at the C=C bond were not formed, and the NOESY spectrum of 7 established its E-configuration evidenced by CH3 and the proton at the double bond interaction.
Conclusion
In conclusion, the Sm-promoted reaction of 1 with methyl 2-bromopropionate led to formation of the substitution product 3 which was different from what could be expected had Sml2 been applied. We associate this course of the reaction with the nature of the Sm reagent, i.e. by generating the rearranged enolate 12. The described unusual variant of fragmentation of 5 under the action of NaHMDS, leading to acyclic lactams 7, is of synthetic interest.
Experimental Section
General
The IR spectra were recorded on a Shimadzu IR Prestige-21 spectrometer from samples prepared as films or mulls in mineral oil. The *H and 13C NMR spectra were recorded on a Bruker AM- 300 (300.13 (*H) and 75.47 (13C) MHz) and Bruker Avance-500 instruments (500.13 1H) and 125.77 (13C) MHz) relative to the residual proton or carbon signals of the deuterated solvent (CHCl3, δ 7.27 ppm; CDCl3, δC 77.00 ppm). The mass spectra (positive electrospray ionization) were obtained on a Shimadzu LCMS-2010EV instrument (samples were injected as solutions in CH3CN with a syringe; eluent acetonitrile-water, 95:5) The progress of reactions was monitored by TLC on Sorbfil plates; spots were detected by treatment with a 10% solution of 4-methoxybenzaldehyde in ethanol containing sulfuric acid.
Sm-promoted Reformat sky reaction of azetidin-2-one 1 with methyl 2-bromopropionate
Methyl 2-bromopropionate (0.35 g, 2.10 mmol) was added drop wise to samarium (0.30 g, 2.10 mmol) (preactivated by heating with 18 mg (0.07 mmol) of iodine) in 7.0 mL dry THF under an argon atmosphere at room temperature. A dark blue color was generated within 0.5-1 h. The azetidin-2-one 1 (0.20 g, 0.70 mmol) was added to the mixture at 0°C. The reaction was stirred for 30 min at the same temperature and then was quenched with saturated solution of NH4Cl. THF was evaporated and the resulting mixture was extracted with ethyl acetate, dried over magnesium sulfate and evaporated to dryness. The residue was purified by column chromatography (silica gel, petroleum ether - ethyl acetate, 8:2 → 7:3) to afford the ether 3 (0.18 g, 70%) and azetidinone 4 (8 mg, 5%).
I. Methyl 2-[(2S,3S)-3-((1R)-1-{[tert-butyl(dimethyl) silyl]oxy}ethyl)-4-oxoazetidin-2-yl]-2(R,S)-methyl-3- oxopentanoate 3: Rf 0.20 (petroleum ether - ethyl acetate, 7:3). White crystal, mp. 84-86 °C. IR, υ, cm-1: 3181, 2921, 1764, 1747, 1716, 1462, 1374, 1252, 1076, 837, 776. Mixture of C2- isomers in a 2:1 ratio (NMR 1H on the intensity of singlet C2-CH3 signals). 1H NMR (500 MHz, CDCl3): δ= 0.05 (s, 6H, CH3), 0.87 (s, 9H, CH3), 1.04 and 1.56* (d, 3H, CH3, J 6.3 Hz), 1.06 and 1.08* (t, 3H, CH3, J 7.2 Hz), 1.38 and 1.43* (s, 3H, CH3), 2.40-2.50 (m, 2H, CH2), 2.88 (br s, 1H, H3') and 2.93* (m, 1H, H3'), 3.75* and 3.78 (s, 3H, OCH3), 4.03*(d, 1H, H2', J 2.0 Hz) and 4.25 (d, 1H, H2', J 2.0 Hz), 4.15* (m, 1H, H1") and 4.20 (dq, 1H, H1", J 2.8, 6.3 Hz), 5.90 (br s, 1H, NH). 13C NMR (125 MHz, CDCl3): -5.07, -4.40 (CH3), 7.99, 8.04 (CH3), 14.59, 16.86 (CH3), 17.91 ((CH3)3C-Si), 22.16, 22.78 (CH3), 25.72 (CH3), 32.46, 32.62 (CH2), 51.66, 52.74 (OCH3), 52.79, 53.03 (C2'), 59.63, 60.17 (C3'), 60.86, 60.92 (C2),64.27,65.28 (CHOTBS), 167.72, 167.97 (CONH), 171.41, 171.72 (CO2Me), 207.56, 207.83 (C=O). MS (ESI): m/z (I, %): 435 (100) [M+Na+MeCN]+, 394 (52) [M+Na]+, 372 (14) [M+H]+.
A. Major diastereoisomer 3: Sample of 90% purity was obtained by repeated column chromatography on the SiO2 of diastereomeric mixture 3 from the previous experiment. White crystals, mp. 68-70 oC, [αϕD -26.8o (c 1.00, CH2cl2)lH NMR (500 MHz, CDC13) δAAA: 0.05 (s, 6H, CH3), 0.87 (s, 9H, CH3),04 (d, 3H, CH3, J 6.4 Hz), 1.06 (t, 3H, CH3, J 7.2 Hz), 1.38 (s, 3H, CH3), 2.42-2.48 (m, 2H, CH2), 2.88 (t, 1H, H3', J 2.3 Hz), 3.78 (s, 3H, OCH3), 4.21 (dq, 1H, CH-OSi, J 2.3, J 6.3 Hz), 4.25 (d, 1H, H2', J 2.3 Hz), 5.80 (br. s, 1H, NH). 13C NMR (125 MHz, CDC13) 5: -5.03, -4.34 (CH3), 8.09 (CH3), 14.61 (CH3), 17.96 ((CH3)3C-Si), 22.20 (CH3), 25.76 (CH3), 32.65 (CH2), 51.68 (OCH3), 52.81 (C2'), 59.65 (C3'), 60.90 (C2), 64.29 (CHOTBS), 168.01 (CONH), 171.76 (CO2Me), 207.90 (C=O).
II.(3S)-3-((1R)-1-{[tert-butyl(dimethyl)silyl]oxy} ethyl)azetidin-2-one 4: Rf 0.19 (petroleum ether - ethyl acetate, 7:3). -71.6o (c 1.00, CHC13).[α]ϕD IR, υ, cm-1: 3183, 2950, 1745, 1463,1372,1073.1H NMR (300 MHz, CDC13) δ: 0.07 (s, 6H, CH3), 0.87 (s, 9H, CH3), 1.20 (d, 3H, CH3, J 6.2 Hz), 3.22 (m, 1H, CHA), 3.29 (t, 1H, H3, J 5.1 Hz), 3.34 (m, 1H, CHB), 4.21 (m, 1H, HI'), 5.66 (br. s, 1H, NH). 13C NMR (125 MHz, CDC13) δ: δ;-5.05, --4.33 (CH3), 17.89 ((CH3)3C-Si), 22.48 (CH3), 25.72 (CH3), 37.62 (CH2), 59.22 (C3), 65.41 (CHOTBS), 169.61 (CONH).
Methyl 2-[(2S,3S)-3-((1R)-1-{[tert-butyl(dimethyl) silyl]oxy}ethyl)-1-(2-methoxy-2-oxoethyl)-4- oxoazetidin-2-yl]-2-(R,5)-methyl-3-oxopentanoate 5
Solution of NaHMDS (1 M solution in THF, 0.36 mL, 0.36 mmol) was added to a solution of compound 3 (0.1 g, 0.27 mmol) and methyl bromoacetate (0.07 g, 0.45 mmol) in 5 ml of anhydrous THF in an argon atmosphere at -78 °C. The reaction mixture was stirred for 1 h (TLC) at the same temperature, and was quenched by saturated aqueous NH4Cl solution (3 mL). THF was evaporated, the aqueous layer was extracted with ethyl acetate, the combined organic extract was washed with saturated brine, dried with MgSO4, filtered, and concentrated in vacuo. The residue was purified by column chromatography (silica gel, petroleum ether - ethyl acetate, 8:2) to afford lactam 5 (0.075 g, 60%) as oily liquid. Rf 0.30 (petroleum ether-ethyl acetate, 7:3). A mixture of C2-diastereomers in a 2:1 ratio. IR, υ, cm-1: 2955, 1768, 1751, 1714, 1437, 1257, 1208, 1114, 1076, 838, 778. *H NMR (500 MHz, CDC13) δ: 0.01, 0.015, 0.02 (s, 6H, CH3), 0.87 (s, 9H, CH3), 1.03 and 1.08* (t, 3H, CH3, J 7.2 Hz), 1.06 and 1.23* (d, 3H, CH3, J 6.1 Hz), 1.42* and 1.53 (s, 3H, CH3), 2.332.50 (m, 2H, CH2), 2.93 (d.d, 1H, H3', J 2.0, 3.7 Hz) and 2.97* (d.d, 1H, H3', J 1.2, 7.0 Hz), 3.67 (s, 3H, OCH3), 3.71* (s, 3H, OCH3), 3.75* (s, 3H, OCH3), 3.96* (d, 1H, HA, J 17.7 Hz) and 4.08 (d, 2H, HB, J 17.7 Hz), 4.12-4.20 (m, 1H, H1"), 4.25* and 4.42 (d, 1H, H2', J 2.0 Hz). 13C NMR (125 MHz, CDC13) δ: -4.58, -4.39 (CH3), 8.11, 8.25 (CH3), 14.39, 17.31 (CH3), 17.84, 17.87 ((CH3)3C-Si), 22.00, 22.74 (CH3), 25.76, 25.81 (CH3), 32.35, 32.43 (CH2), 42.63, 43.63 (CH2), 51.96, 52.14 (OCH3), 52.75, 52.83 (C2'), 57.22, 58.39 (OCH3), 59.20, 60.18 (C3'), 61.32, 61.43 (C2), 65.12, 66.83 (CHOTBS),168.27, 168.86 (CONH), 169.26, 171.28 (CO2Me), 207.63, 207.91 (C=O). MS (ESI): m/z (I, %): 466 (100) [M+Na]+, 507 (26) [M+Na+MeCN]+.
Dimethyl{[(2R,S,3Z)-2-((1R)-1-{[tert- butyl(dimethyl)silyl]-oxy}ethyl)-4-methyl-5-oxohept- 3-enoyl]amino}malonate 7
To a solution of the azetidinone 5 (0.06 g, 0.13 mmol) in anhydrous THF (4mL) was added NaHMDS (1 M in THF, 0.10 mL, 0.10 mmol) at -78 °C in argon atmosphere. The reaction mixture was stirred for 0.5 h (TLC) at the same temperature, and was quenched by saturated aqueous NH4Cl solution (3 mL).THF was evaporated, the aqueous layer was extracted with ethyl acetate, the combined organic extract was washed with saturated brine, dried with MgSO4, filtered, and concentrated in vacuo. The residue was purified by column chromatography (silica gel, petroleum ether - ethyl acetate, 8:2) to afford 7 (0.037 g, 62%) as 1:1 diastereomeric mixture of an oily liquid. Rf 0.30 ((petroleum ether - ethyl acetate, 7:3). IR, υ, cm-1: 3300, 2955, 1758, 1720, 1674, 1509, 1257, 1237, 1114, 978, 836, 777. H NMR (500 MHz, CDC13] <δ: 0.07, 0.08, 0.10 and 0.11 (s, 6H, CH3], 0. 88.and 0.09 (s, 9H, CH3], 1.10 (d.t, 6H, CH3, J 3.1, 7.3 Hz], 1.19 and 1.20 (d, 3H, CH3, J 6.3 Hz), 1.88 (t, 3H, CH3, J 1.5 Hz), 2.602.85 (m, 2H, CH2), 3.35 (m, 1H, H2') and 3.73 (s, 3H, OCH3), 3.80 (s, 3H, OCH3), 4.10 (m, 1H, CH-OSi), 5.25 and 5.27 (d, 1H, H1, J 4.1 Hz], 6.90 (m, 1H, H/], 7.40 (t, 1H, NH, J 7.4 Hz], 13C NMR (125 MHz, CDC13] δ : -5.03, -4.63 (CH3], 7.39, 7.44 (CH3], 13.01 (CH3],17.90 ((CH3]3C-Si], 20.43, 20.47 (CH3], 25.66 (CH3], 34.30, 34.50 (CH2), 51.87, 53.08 (OCH3), 52.96, 53.16 (C2'), 62.03, 62.12 (C2),69.90 (CHOTBS), 131.13 (C4), 135.86 and 135.90 (CH=), 166.49, 167.82 (CONH), 170.35 and 170.38 (CO2Me), 200.97 and 201.48 (C=O). MS (ESI): m/z (I, %): 444 (100) [MH]+.
Acknowledgment
The study was performed under financial support by the Russian Science Foundation (project no. 15-13-00 039).
To know more about Journal of chemistry,
Click here: https://juniperpublishers.com/omcij/index.php
To know more abour juniper Publishers,
click here: https://juniperpublishers.com/index.php
#juniper publishers#juniper publishers group#juniperpublishers#juniper publisher reviews#chemistry#open access journals#Open access Journal of chemistry#chemistry journal
0 notes






