#Blood Coagulation Testing Market
Link
Blood Coagulation Testing Market share Economic Impact, Dynamics and SWOT Analysis Till 2030
0 notes
Text
Blood Coagulation Testing Market: Industry Trends, Revenue, Growth Rate, Impact Factors and Forecast: 2022-2027

Market Overview
Blood Coagulation Testing tests and measures your blood's capacity and ability to clot and the time consumed to clot. This is done to keep track of blood regulation and maintenance so that the body functions without any interference. Blood Coagulation Testing Market share also makes sure to check on your body's healthy functioning without any side effects. When you have access to the functioning of your body and its metabolism, it becomes quite easy to understand the chances of any risks or clotting that could have taken place.
These tests are mostly similar to the blood tests in their conduction, but the results can give you a deep understanding of your body's functioning. This also helps in keeping a check on the real-time metabolism.
The Blood Coagulation Testing Market was estimated at 3,566 million dollars by 2026, sharply increasing at a 5.9% CAGR. This is also predicted to generate a revenue of $1094 million and more by 2026.
The POC or the global point of care coagulation testing devices measure blood coagulation levels. The outbreak of the Covid pandemic harmed the global market growth in this sector and led to a huge decline in the revenue rates of the reports. A study published by the French researchers in the Medical Care Journal Suggested that the patients with Covid-19 who didn't have their blood coagulation tests done were more prone to the infection than those who had it done.
Some of the most common blood coagulation tests include the D-dimer, thromboplastin time (ppt), activated partial thromboplastin time (apt), etc.
Despite the benefits provided by the blood coagulation testing devices, certain factors limit the industry's growth in the global market. This is because of the high-cost associated factors and the unawareness of the procedure of the operating devices.
Market Segmentation
The segmentation of the Blood Coagulation Testing Market is done in several ways. These include the user-end analysis and the device type analysis.
In the device-type segmentation, the coagulation testing devices are fragmented into the monitoring devices, which focus on the anti-coagulation factors, platelet functioning devices, and viscoelastic coagulation monitoring devices.
In the end-user fragmentation of the market segmentation, the centers include hospitals, homecare, and clinics
Homecare comes in use while considering a case with a higher rate of CAGR, which leads to an increase in the awareness of the case in the general public. This is also with regards to various bleeding disorders where the homecare comes in handy. . The hospital and the clinics, however, hold a dominating share of the coagulation testing market due to the increased number of bleeding disorders in the same case.
Regional Analysis
According to a recent geographical stat, the Asia-Pacific region has the maximum blood warming devices dominance. This includes countries like China and India that have their global markets interlinked to the BLOOD COAGULATION TESTING MARKET domains. Countries like Europe and North America follow this demand.
Industry News
The BLOOD COAGULATION TESTING MARKET was estimated at 3,566 million dollars by 2026, which sharply increased at a 5.9% CAGR. This is also predicted to generate a revenue of $1094 million and more by 2026.
The key players in the blood coagulation testing market are:
Danaher
Abbott Laboratories
Bios stem S.A.
Helena Laboratories
Siemens
Hoffmann-La Roche
laboratory Corporation of America Holding
Sienco, Inc
A&T Corporation
About Market Research Future:
At Market Research Future (MRFR), we enable our customers to unravel the complexity of various industries through our Cooked Research Report (CRR), Half-Cooked Research Reports (HCRR), & Consulting Services. MRFR team have supreme objective to provide the optimum quality market research and intelligence services to our clients.
Contact us:
Market Research Future (part of Wantstats Research and Media Private Limited),
99 Hudson Street, 5Th Floor,
New York, New York 10013
United States of America
+1 628 258 0071
Email: [email protected]
0 notes
Text
Horseshoe crab blood is vital for testing intravenous drugs, but new synthetic alternatives could mean pharma won’t bleed this unique species dry
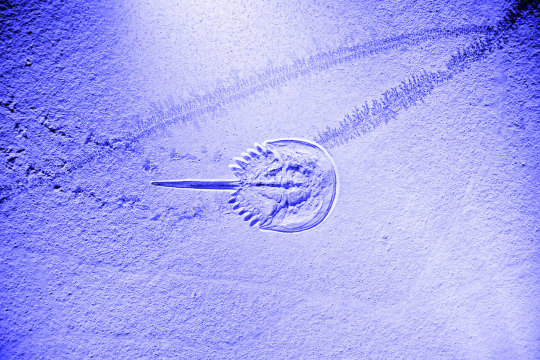
- By Kristoffer Whitney , Jolie Crunelle , Rochester Institute of Technology , The Conversation -
If you have ever gotten a vaccine or received an intravenous drug and did not come down with a potentially life-threatening fever, you can thank a horseshoe crab (Limulus polyphemus).
How can animals that are often called living fossils, because they have barely changed over millions of years, be so important in modern medicine? Horseshoe crab blood is used to produce a substance called limulus amebocyte lysate, or LAL, which scientists use to test for toxic substances called endotoxins in intravenous drugs.
These toxins, produced by bacteria, are ubiquitous in the environment and can’t be removed simply through sterilization. They can cause a reaction historically referred to as “injection fever.” A strong concentration can lead to shock and even death.
Identifying LAL as a highly sensitive detector of endotoxins was a 20th-century medical safety breakthrough. Now, however, critics are raising questions about environmental impacts and the process for reviewing and approving synthetic alternatives to horseshoe crab blood.
We study science, technology and public policy, and recently published a white paper examining social, political and economic issues associated with using horseshoe crabs to produce LAL. We see this issue as a test case for complicated problems that cut across multiple agencies and require attention to both nature and human health.
youtube
Protecting horseshoe crabs will require persuading the heavily regulated pharmaceutical industry to embrace change.
An ocean solution
Doctors began injecting patients with various solutions in the mid-1800s, but it was not until the 1920s that biochemist Florence Seibert discovered that febrile reactions were due to contaminated water in these solutions. She created a method for detecting and removing the substances that caused this reaction, and it became the medical standard in the 1940s.
Known as the rabbit pyrogen test, it required scientists to inject intravenous drugs into rabbits, then monitor the animals. A feverish rabbit meant that a batch of drugs was contaminated.
The LAL method was discovered by accident. Working with horseshoe crabs at the Marine Biological Laboratory at Woods Hole, Massachusetts, in the 1950s and ’60s, pathobiologist Frederik Bang and medical researcher Jack Levin noticed that the animals’ blue blood coagulated in a curious manner. Through a series of experiments, they isolated endotoxin as the coagulant and devised a method for extracting LAL from the blood. This compound would gel or clot nearly instantaneously in the presence of fever-inducing toxins.
Academic researchers, biomedical companies and the U.S. Food and Drug Administration refined LAL production and measured it against the rabbit test. By the 1990s, LAL was the FDA-approved method for testing medicines for endotoxin, largely replacing rabbits.
Producing LAL requires harvesting horseshoe crabs from oceans and beaches, draining up to 30% of their blood in a laboratory and returning the live crabs to the ocean. There’s dispute about how many crabs die in the process – estimates range from a few percent to 30% or more – and about possible harmful effects on survivors.
Today there are five FDA-licensed LAL producers along the U.S. East Coast. The amount of LAL they produce, and its sales value, are proprietary.
Bait versus biotech
As biomedical LAL production ramped up in the 1990s, so did harvesting horseshoe crabs to use as bait for other species, particularly eel and whelk for foreign seafood markets. Over the past 25 years, hundreds of thousands – and in the early years, millions – of horseshoe crabs have been harvested each year for these purposes. Combined, the two fisheries kill over half a million horseshoe crabs every year.
There’s no agreed total population estimate for Limulus, but the most recent federal assessment of horseshoe crab fisheries found the population was neither strongly growing nor declining.
Conservationists are worried, and not just about the crabs. Millions of shorebirds migrate along the Atlantic coast, and many stop in spring, when horseshoe crabs spawn on mid-Atlantic beaches, to feed on the crabs’ eggs. Particularly for red knots – a species that can migrate up to 9,000 miles between the tip of South America and the Canadian Arctic – gorging on horseshoe crab eggs provides a critical energy-rich boost on their grueling journey.
Red knots were listed as threatened under the Endangered Species Act in 2015, largely because horseshoe crab fishing threatened this key food source. As biomedical crab harvests came to equal or surpass bait harvests, conservation groups began calling on the LAL industry to find new sources.
Biomedical alternatives
Many important medicines are derived from living organisms. Penicillin, the first important antibiotic, was originally produced from molds. Other medicines currently in use come from sources including cows, pigs, chickens and fish. The ocean is a promising source for such products.
When possible, synthesizing these substances in laboratories – especially widely used medications like insulin – offers many benefits. It’s typically cheaper and more efficient, and it avoids putting species at risk, as well as addressing concerns some patients have about using animal-derived medical products.
In the 1990s, researchers at the National University of Singapore invented and patented the first process for creating a synthetic, endotoxin-detecting compound using horseshoe crab DNA and recombinant DNA technology. The result, dubbed recombinant Factor C (rFC), mimicked the first step in the three-part cascade reaction that occurs when LAL is exposed to endotoxin.
Later, several biomedical firms produced their own versions of rFC and compounds called recombinant cascade reagents (rCRs), which reproduce the entire LAL reaction without using horseshoe crab blood. Yet, today, LAL remains the dominant technology for detecting endotoxins in medicine.

A sample of horseshoe crab blood. Florida Fish and Wildlife Commission, CC BY-NC-ND
The main reason is that the U.S. Pharmacopeia, a quasi-regulatory organization that sets safety standards for medical products, considers rFC and rCR as “alternative” methods for detecting endotoxins, so they require case-by-case validation for use – a potentially lengthy and expensive process. The FDA generally defers to the U.S. Pharmacopeia.
A few large pharmaceutical companies with deep pockets have committed to switching from LAL to rFC. But most drug producers are sticking with the tried-and-true method.
Conservation groups want the U.S. Pharmacopeia to fully certify rFC for use in industry with no extra testing or validation. In their view, LAL producers are stalling rFC and rCR approval to protect their market in endotoxin detection. The U.S. Pharmacopeia and LAL producers counter that they are doing due diligence to protect public health.
Change in the offing
Change may be coming. All major LAL producers now have their own recombinant products – a tacit acknowledgment that markets and regulations are moving toward Limulus-free ways to test for endotoxins.
Atlantic fisheries regulators are currently considering new harvest limits for horseshoe crabs, and the U.S. Pharmacopeia is weighing guidance on recombinant alternatives to LAL. Public comments will be solicited over the winter of 2024, followed by U.S. Pharmacopeia and FDA review.
Even if rFC and rCR don’t win immediate approval, we believe that collecting more complete data on horseshoe crab populations and requiring more transparency from the LAL industry on how it handles the crabs would represent progress. So would directing medical companies to use recombinant products for testing during the manufacturing process, while saving LAL solely for final product testing.
Making policy on complex scientific issues across diverse agencies is never easy. But in our view, incremental actions that protect both human health and the environment could be important steps forward.
Kristoffer Whitney, Associate Professor of Science, Technology and Society, Rochester Institute of Technology and Jolie Crunelle, Master's Degree Student in Science, Technology, and Public Policy, Rochester Institute of Technology
This article is republished from The Conversation under a Creative Commons license. Read the original article.
--
Read Also
The FDA no longer mandates all drugs to be tested on animals before being tested on humans
#animals#animal welfare#biotech#pharma#clinical trials#medtech#health#blood#medicine#animal testing#horseshoe crab
2 notes
·
View notes
Text
Hemostasis/ Coagulation Analyzer Market Will Grow at Highest Pace Owing to Point-of-Care Diagnostics
Hemostasis and coagulation analyzers are medical devices used to test coagulation factors in blood plasma. They help in monitoring blood coagulation disorders and management of anticoagulation therapies. Hemostasis and coagulation analyzers detect abnormalities related to hemostasis that may lead to excessive bleeding or thrombosis. Technological advancements have improved portability and results time of these analyzers enabling point-of-care diagnosis.
The Global Hemostasis/ Coagulation Analyzer Market is estimated to be valued at US$ 6.47 Bn in 2024 and is expected to exhibit a CAGR of 5.8% over the forecast period 2024 to 2031.
Key Takeaways
Key players operating in the Hemostasis/ Coagulation Analyzer Market Growth are Horiba Medical, Siemens Healthineers AG, Agappe Diagnostics Ltd, NIHON KOHDEN CORPORATION, Sysmex Corporation, F. Hoffmann-La Roche Ltd, Stago Group, Maccura Biotechnology Co., Ltd., Thermo Fisher Scientific Inc., Haemonetics Corporation, Genrui Biotech Co., Ltd., BIOLABO S.A.S, Erba Mannheim, Sclavo Diagnostics International, and Helena Laboratories Corporation.
The growing demand for point-of-care hemostasis testing is driving the market. Portable analyzers provide rapid results at the patient's bedside, ER, OR, and other locations to expedite clinical decision making.
Technological advancements like dry chemistry methods, microfluidics, and automated sample-to-result technologies are reducing complex manual tasks. New devices have enhanced throughput, usability, and data management features for busy healthcare settings.
Market Trends
Point-of-care testing trend continues to grow for decentralized healthcare models. New devices offer miniaturized assaysoutside conventional laboratories directly at the site of patient care.
Artificial intelligence and machine learning are being applied for tasks like automatic result interpretation, quality control, predictive maintenance, and remote diagnostics support. This helps maximize analyzer uptime for uninterrupted patient testing.
Market Opportunities
Emerging markets show strong growth potential driven by increasing access to healthcare and focus on non-communicable diseases. Local product assembly, innovative financingmodels, and regulatory approval strategies can help manufacturers tap opportunities.
Integration of hemostasis testingonto general chemistry and coagulation analyzer platformsprovides a one-stop solution for routine and specialized testing. This consolidatestesting workflow while reducing costs per reportable result.
Get More Insights On This Topic: Hemostasis/ Coagulation Analyzer Market
0 notes
Text
Middle East IVD Market - Opportunity Analysis and Industry Forecast (2024-2031)
Meticulous Research®, a leading global market research company, published a research report titled, ‘Middle East IVD Market by Offering (Kits, Software), Technology (Immunoassay, Molecular Diagnostics [PCR, NGS, Microarray], Rapid Tests, Biochemistry), Application (Infectious Diseases, Oncology), Diagnostic Approach (Lab) - Forecast to 2031.’
According to this latest publication from Meticulous Research®, the Middle East In Vitro Diagnostics (IVD) market is expected to reach $2.15 billion by 2031 at a CAGR of 4.1% from 2024 to 2031. The growth of the Middle East IVD market is attributed to the rising prevalence of chronic and infectious diseases, the growing demand for point-of-care (PoC) and rapid diagnostics, rising healthcare expenditures, growth in the aging population, and rising awareness about healthcare. However, high prices for IVD tests and the variance in test results observed in rapid IVD tests restrain the growth of the IVD market.
The rising awareness regarding early disease diagnosis and increasing inclination toward personalized medicine in the UAE and Saudi Arabia are expected to create growth opportunities for the players operating in this market. However, the concerns pertaining to false positive results in immunoassays and POC are a major challenge for market growth.
Key Players
The key players operating in the Middle East IVD market are Abbott Laboratories (U.S.), Becton, Dickinson and Company (U.S.), BioMérieux S.A. (France), Danaher Corporation (U.S.), F. Hoffmann-La Roche (Switzerland), QIAGEN N.V. (Netherlands), Siemens Healthineers AG (Germany), Thermo Fisher Scientific Inc. (U.S.), Bio-Rad Laboratories, Inc. (U.S.), Illumina, Inc. (U.S.), QuidelOrtho Corporation. (U.S.), Agilent Technologies, Inc. (U.S.), and DiaSorin S.p.A. (Italy).
Middle East IVD Market: Future Outlook
The Middle East IVD market is segmented by Offering (Kits & Reagents, Instruments, and Software & Services), Technology (Immunoassay/Immunochemistry, Biochemistry/Clinical Chemistry, Molecular Diagnostics, Point of Care (POC) Diagnostics, Whole Blood Glucose Monitoring, Microbiology, Hematology, Coagulation/Hemostasis, Urinalysis, and Other Technologies), Application (Infectious Diseases, Oncology, Diabetes, Cardiology, Nephrology, Autoimmune Disorders, and Other Applications), Diagnostic Approach (Lab Testing, OTC/Self-testing, and Point of Care Testing), and End User (Diagnostic Laboratories, Hospitals & Clinics, Home Healthcare, and Other End Users), and Countries. The study also evaluates industry competitors and analyzes their market share at the country level.
Among all offerings studied in this report, the kits & reagents segment is expected to register the highest CAGR over the forecast period. The highest CAGR of this segment is attributed to the frequent use of reagents and kits in the detection of various chronic and infectious diseases, the wide commercial availability of a diverse range of reagents and consumables for the diagnosis of various diseases, the increase in the volume of testing for infectious diseases such as HIV and influenza, and the growing awareness about self-testing among the general population. According to UNAIDS, global HIV prevalence is decreasing, but the Middle East and North Africa are one of the few regions where new HIV infections are increasing at a rapid rate. In 2022, only 67% of people living with HIV knew their HIV-positive status.
Among all technologies studied in this report, in 2024, the molecular diagnostic segment is expected to account for the largest share of the Middle East IVD market. The large market share of this segment is attributed to the high prevalence of communicable and non-communicable diseases requiring molecular diagnostics (molecular diagnostic tests give more access to high-volume testing in less time and better specificity) and increasing the adoption of molecular diagnostic techniques in hospitals and laboratories.
Among all applications studied in this report, in 2024, the infectious disease segment is expected to account for the largest share of the Middle East IVD market. Infectious diseases are further categorized into respiratory infections (excluding influenza), hepatitis, HIV, HAIs, tropical diseases, influenza, sexually transmitted diseases (STDs), and other infectious diseases. The increasing prevalence of seasonal influenza and sexually transmitted diseases (STDs) is increasing the demand for IVD testing. For instance, people aged over 65 years account for approximately 50-70% of hospitalizations & 90% of influenza-related deaths.
Among all diagnostic approaches studied in this report, the point-of-care testing segment is expected to grow at the highest CAGR during the forecast period. The growth of the POC testing segment is attributed to the increasing prevalence of chronic diseases, the launch of PoC tests by market players, and the lack of skilled technicians for performing lab tests. Additionally, the launch of PoC testing in the region is contributing to the growth of this segment.
Among all end users studied in this report, in 2024, the hospitals & clinics segment is expected to account for the largest share of the Middle East IVD market. Hospitals and clinics perform a wide range of tests to diagnose various medical conditions, including communicable and non-communicable diseases. Hospitals in urban and rural areas provide large populations with access to diagnosis. Also, there was a significant increase in hospital admissions due to a rise in the rate of infectious diseases, which boosted the demand for diagnostic products.
Geographic Review
This research report comprehensively analyzes major countries: Saudi Arabia, UAE, Qatar, Kuwait, Oman, Israel, and the rest of the Middle East. In 2024, Saudi Arabia is expected to account for the largest share of the Middle East IVD market. The largest share of the country is attributed to the growing aging, rising chronic and infectious diseases, and the increasing adoption of self-testing. Additionally, rising healthcare expenditure is increasing the demand for IVD. The healthcare expenditure in Saudi Arabia is rising. The Government of Saudi Arabia has implemented various reforms under the “Vision 2030” strategy to improve the business environment and sectors, including healthcare. Saudi Arabia spent an estimated 6.6% of its GDP on healthcare expenditure in 2021, which is 3.7% higher than the healthcare expenditure a decade ago. Healthcare expenditure in Saudi Arabia is expected to increase at an annual average of 3.0% from 2022 to 2025 to meet “Vision 2030” goals.
Download Sample Report Here @ https://www.meticulousresearch.com/download-sample-report/cp_id=5764
Key questions answered in the report:
Which are the high-growth market segments in terms of the offering, technology, application, diagnostic approach, end user, and country?
What was the historical market for IVD testing in the Middle East?
What are the market forecasts and estimates for the period 2024–2031?
What are the major drivers, restraints, challenges, and opportunities in the Middle East IVD market?
Who are the major players in the Middle East IVD market?
Contact Us:
Meticulous Research®
Email- [email protected]
Contact Sales- +1-646-781-8004
Connect with us on LinkedIn- https://www.linkedin.com/company/meticulous-research
#MiddleEastInVitroDiagnosticsMarket#MiddleEastIVDMarket#RapidDiagnostics#InVitroDiagnostics#IVD#Immunodiagnostics#PointofCareDiagnostics#MolecularDiagnostics
0 notes
Text
D-dimer Testing Market to hit USD 2 Bn by 2032, says Global Market Insights Inc.

D-Dimer Testing Market size is predicted to register over USD 2 billion by 2032. The increasing prevalence of conditions, such as deep vein thrombosis (DVT), pulmonary embolism (PE), and disseminated intravascular coagulation (DIC) will drive the industry progression.
Request for a sample of this research report @ https://www.gminsights.com/request-sample/detail/5972
As per the Centre of Disease Control and Prevention (CDC), the number of individuals annually impacted by deep vein thrombosis (DVT) or pulmonary embolism (PE) reached 900,000 people in the U.S. These conditions often necessitate D-Dimer testing as part of the diagnostic process to assess the likelihood of thrombotic events. The rising aging population, associated with obesity and sedentary habits, is also contributing to the growing incidence of thrombotic disorders.
Rising advancements in healthcare infrastructure, particularly in emerging economies, are boosting accessibility to diagnostic services, including D-Dimer testing. There is a growing awareness among healthcare professionals about the utility of this testing in diagnosing thrombotic disorders at an early stage. The integration of novel technologies, such as point-of-care testing and automation in laboratory settings to enhance the efficiency and accuracy of D-Dimer assays will add to the market gains.
Rising usage to treat pulmonary embolism
D-Dimer testing market from the pulmonary embolism (PE) application segment is slated to record high demand by 2032, as it represents a life-threatening condition characterized by the blockage of arteries in the lungs, often resulting from blood clots originating in other body parts. D-Dimer testing helps in the assessment of suspected cases of pulmonary embolism, aiding clinicians in ruling out or confirming the presence of thrombotic events. The rising incidence of prolonged immobility, surgery, and certain medical conditions will also contribute to the increased demand for PE diagnosis.
Growing presence in hospitals
The hospitals segment in the D-dimer testing market is poised to generate notable revenues during 2024 and 2032, due to their higher popularity as primary hubs for patient care, diagnosis, and treatment. With rising burden of thrombotic disorders globally, hospitals witness a steady influx of patients with symptoms like deep vein thrombosis (DVT) and pulmonary embolism. Hospitals also often house advanced laboratory facilities equipped with automated testing platforms and skilled personnel for facilitating efficient and high-throughput D-Dimer testing.
Asia Pacific to emerge as a lucrative market
The Asia Pacific D-dimer testing market is touted to surge at a rapid pace from 2024 to 2032, attributed to the increasing adoption of western lifestyle habits coupled with rising urbanization and sedentary lifestyles. The expanding geriatric population across Asia Pacific countries, particularly in Japan, China, and India, is predisposed to a higher incidence of thrombotic events. The improving healthcare infrastructure and growing investments in medical R&D activities in countries like South Korea and Singapore are facilitating greater accessibility to advanced diagnostic technologies.
Request for Report Customization @ https://www.gminsights.com/roc/5972
D-Dimer Testing Industry Participants
Some major companies in the global D-Dimer testing market include Siemens Healthcare GmbH, Abbott Laboratories, Thermo Fisher Scientific Inc., BioMedica Diagnostics, Unbound Medicine Inc biomérieux SA, Quidel Corporation, Diazyme Laboratories Inc. (General Atomics), FHoffmann-La Roche Ltd., HORIBA Ltd., Sekisui Diagnostics LLC (Sekisui Chemical Co. Ltd.), and Werfen.
These firms are focusing on partnership ventures to widen their global presence and customer base. For instance, in May 2022, the assay, developed by Horiba Medical, successfully detected venous thromboembolism (VTE) with excellent accuracy, reliability, and turnaround time on the manufacturer's Yumizen G800 fully automated hemostasis analyzer.
#Ddimer#BloodClotTesting#Thrombosis#CoagulationTesting#CardiovascularHealth#MedicalDiagnosis#Hemostasis#DiagnosticTesting#ClotDetection#LaboratoryMedicine
1 note
·
View note
Text
Coagulation Factor Deficiency Market Potential Growth Opportunities and Competitive Landscape Report to 2033
Market Definition
Coagulation factor deficiency is a condition in which the body does not produce enough of one or more of the proteins responsible for blood clotting. This can lead to excessive bleeding or a tendency to bleed for longer periods of time. Coagulation factor deficiencies can be caused by a variety of conditions, including inherited genetic disorders, acquired diseases, or medications.
Market Dynamics
The most common inherited coagulation factor deficiency is hemophilia A, which is caused by mutations in the gene responsible for producing factor VIII. This condition leads to a deficiency of factor VIII, which is necessary for the normal clotting of blood. People with hemophilia A may experience excessive bleeding from minor cuts or bruises and may require frequent transfusions to replace the missing factor VIII.
Other inherited coagulation factor deficiencies include factor IX deficiency (hemophilia B), factor XI deficiency (hemophilia C), and factor XIII deficiency. These conditions are all caused by mutations in the genes responsible for producing the respective clotting factors. People with these conditions may also experience excessive bleeding and may require transfusions.
Acquired coagulation factor deficiencies can also occur. These are usually caused by diseases such as liver disease or vitamin K deficiency. Liver disease can lead to a deficiency of multiple clotting factors, while vitamin K deficiency can lead to a deficiency of factor II (prothrombin). Both of these conditions can lead to excessive bleeding.
To Know More@��https://www.globalinsightservices.com/reports/coagulation-factor-deficiency-market/?utm_id=1014
Finally, certain medications can also cause coagulation factor deficiencies. Warfarin, for example, is a blood thinner that works by interfering with the production of clotting factors. People taking warfarin may experience excessive bleeding and may require transfusions to replace the missing clotting factors.
Key Trends
Coagulation Factor Deficiency (CFD) technology is a rapidly evolving field of medical science that focuses on the diagnosis, treatment, and prevention of bleeding disorders caused by a deficiency in specific clotting factors. CFD technology is an important tool for physicians, as it can help detect, treat, and prevent the serious consequences of bleeding disorders. As such, it is important to understand the key trends in CFD technology in order to stay up to date with the latest advances in this field.
The first key trend in CFD technology is the development of new diagnostic tests. Diagnostic tests are an essential part of the treatment process for CFD, as they can help identify the specific clotting factor deficiency and inform treatment decisions. Recent advances in CFD technology have led to the development of more accurate and sensitive tests, such as the prothrombin time (PT) test, which can detect a deficiency in prothrombin, the most important clotting factor. Additionally, new tests are being developed to detect more rare clotting factor deficiencies, such as factor V Leiden and factor VIII deficiency.
The second key trend in CFD technology is the development of new treatments. Treatment for CFD primarily consists of replacement therapy, in which the missing clotting factor is replaced with a medication or a blood product. Recent advances in CFD technology have led to the development of new medications and blood products, such as recombinant factor VIII and recombinant factor IX, which are more effective and have fewer side effects than traditional treatments. Additionally, gene therapy is being explored as a potential treatment for CFD, with promising results in animal models.
The third key trend in CFD technology is the development of new preventive measures. Preventive measures are essential for reducing the risk of bleeding complications in individuals with CFD. Recent advances in CFD technology have led to the development of new preventive measures, such as the use of low-dose aspirin to reduce the risk of bleeding in individuals with factor V Leiden and factor VIII deficiency. Additionally, new medications are being developed to reduce the risk of bleeding in individuals with other clotting factor deficiencies.
Key Drivers
Coagulation factor deficiency is a medical condition in which the body does not produce enough of the proteins that are necessary for normal blood clotting. It can lead to a variety of serious complications including excessive bleeding, anemia, and even death in some cases. Treatment for this condition typically involves replacing lost or missing proteins in the form of a medication or transfusion.
The global coagulation factor deficiency market is driven by a number of factors. These include increasing prevalence of blood disorders, rising geriatric population, growing awareness of the condition, increasing healthcare expenditure, and the availability of novel treatments.
Request Sample@ https://www.globalinsightservices.com/request-sample/GIS26274/?utm_id=1014
The prevalence of coagulation factor deficiency is increasing due to a variety of factors. These include lifestyle changes, such as poor diet and lack of exercise, as well as environmental factors, such as exposure to toxins. This has led to a rise in the number of patients suffering from this condition. In addition, the aging population is also contributing to the increasing prevalence of this condition, as the risk of developing it increases with age.
The rising awareness of coagulation factor deficiency is also driving the market. Organizations such as the World Health Organization (WHO) are raising awareness of the condition and the need for proper diagnosis and treatment. This is leading to an increase in the number of patients seeking treatment for this condition.
In addition, the increasing healthcare expenditure is also driving the market. Governments and private organizations are investing more in healthcare, which is leading to an increase in the availability of treatments for this condition. This is allowing more patients to receive the treatments they need.
Finally, the availability of novel treatments is also driving the market. Advances in medical technology have led to the development of new treatments for coagulation factor deficiency, such as gene therapy and artificial blood products. These treatments are providing patients with more options for managing their condition.
Research Objectives
Estimates and forecast the overall market size for the total market, across product, service type, type, end-user, and region
Detailed information and key takeaways on qualitative and quantitative trends, dynamics, business framework, competitive landscape, and company profiling
Identify factors influencing market growth and challenges, opportunities, drivers and restraints
Identify factors that could limit company participation in identified international markets to help properly calibrate market share expectations and growth rates
Trace and evaluate key development strategies like acquisitions, product launches, mergers, collaborations, business expansions, agreements, partnerships, and R&D activities
Thoroughly analyze smaller market segments strategically, focusing on their potential, individual patterns of growth, and impact on the overall market
To thoroughly outline the competitive landscape within the market, including an assessment of business and corporate strategies, aimed at monitoring and dissecting competitive advancements.
Identify the primary market participants, based on their business objectives, regional footprint, product offerings, and strategic initiatives
Request Customization@ https://www.globalinsightservices.com/request-customization/GIS26274/?utm_id=1014
Market Segmentation
The market can be segmented by product type, end-user, distribution channel, and region. By type, the market can be divided into Coagulation Factor IX and Coagulation Factor XIII & others. By Application, the market can be divided into Hospitals and Clinics and Research Laboratories & academic Institutions. By region, the market is divided into North America, Europe, Asia-Pacific, and the Rest of the World.
Key Players
The market includes players such as Pfizer Inc.(U.S.), Bayer AG(Germany), Baxter(U.S.), Biogen(U.S.), Octapharma AG (Switzerland), CSL Limited (Australia), Novo Nordisk A/S(Denmark), Mylan N.V.(U.S.), Sanofi (France), and Zydus Cadila(India).
Buy your copy here@ https://www.globalinsightservices.com/checkout/single_user/GIS26274/?utm_id=1014
Research Scope
Scope – Highlights, Trends, Insights. Attractiveness, Forecast
Market Sizing – Product Type, End User, Offering Type, Technology, Region, Country, Others
Market Dynamics – Market Segmentation, Demand and Supply, Bargaining Power of Buyers and Sellers, Drivers, Restraints, Opportunities, Threat Analysis, Impact Analysis, Porters 5 Forces, Ansoff Analysis, Supply Chain
Business Framework – Case Studies, Regulatory Landscape, Pricing, Policies and Regulations, New Product Launches. M&As, Recent Developments
Competitive Landscape – Market Share Analysis, Market Leaders, Emerging Players, Vendor Benchmarking, Developmental Strategy Benchmarking, PESTLE Analysis, Value Chain Analysis
Company Profiles – Overview, Business Segments, Business Performance, Product Offering, Key Developmental Strategies, SWOT Analysis.
With Global Insight Services, you receive:
10-year forecast to help you make strategic decisions
In-depth segmentation which can be customized as per your requirements
Free consultation with lead analyst of the report
Infographic excel data pack, easy to analyze big data
Robust and transparent research methodology
Unmatched data quality and after sales service
Contact Us:
Global Insight Services LLC
16192, Coastal Highway, Lewes DE 19958
E-mail: [email protected]
Phone: +1-833-761-1700
Website: https://www.globalinsightservices.com/
0 notes
Text
0 notes
Text
Unveiling the Dynamics of the Global Hematology Testing Market

Introduction:
The field of hematology plays a crucial role in understanding and diagnosing various blood-related disorders. Hematology testing has evolved significantly over the years, with technological advancements paving the way for more accurate and efficient diagnostic methods. In this blog, we will delve into the dynamics of the Global Hematology Testing Market, exploring the key trends, challenges, and opportunities that define this rapidly growing sector.
Market Overview:
The Global Hematology Testing Market is witnessing unprecedented growth, driven by factors such as the rising prevalence of blood disorders, increasing geriatric population, and the continuous quest for innovative diagnostic solutions. Hematology testing encompasses a broad spectrum of tests, ranging from complete blood count (CBC) to more specialized tests like coagulation testing and blood typing.
Key Market Trends:
Automation and Integration: The demand for automated hematology analyzers is on the rise as laboratories seek to enhance efficiency and reduce turnaround times. Integrated systems that combine multiple testing functions are becoming increasingly popular, allowing for seamless workflow and improved accuracy.
Point-of-Care Testing (POCT): The shift towards decentralized testing is evident in the growing adoption of POCT devices. These compact, user-friendly instruments provide rapid results, enabling healthcare professionals to make quick decisions and initiate timely interventions.
Technological Advancements: The development of advanced technologies, including flow cytometry and molecular diagnostics, is revolutionizing hematology testing. These innovations not only improve the precision of diagnostics but also open doors to a more comprehensive understanding of blood-related disorders.
Experience Exponential Growth: Secure Your Sample Report and Dominate the Global Hematology Testing Market @ https://bisresearch.com/requestsample?id=778&type=download
Challenges in the Market:
Cost Constraints: The initial investment required for state-of-the-art hematology testing equipment can be a deterrent, especially for smaller healthcare facilities. The challenge lies in striking a balance between technological advancement and cost-effectiveness.
Regulatory Compliance: The hematology testing market is subject to stringent regulatory frameworks. Navigating these compliance requirements poses challenges for manufacturers and healthcare providers, necessitating a keen focus on quality control and assurance.
Opportunities on the Horizon:
Emerging Markets: Developing regions present untapped opportunities for growth in the hematology testing market. Increasing awareness, improving healthcare infrastructure, and rising disposable incomes contribute to the expansion of the market in these areas.
Personalized Medicine: The integration of hematology testing into the realm of personalized medicine is an exciting prospect. Tailoring treatment strategies based on individual patient profiles can significantly improve outcomes and redefine the landscape of blood disorder management.
Conclusion:
As the Global Hematology Testing Market continues to evolve, driven by technological advancements and a growing awareness of the importance of early and accurate diagnostics, the future holds immense promise. Overcoming challenges and leveraging emerging opportunities will be crucial for stakeholders in this dynamic and vital sector, ultimately contributing to better patient outcomes and a healthier global population.
#Global Hematology Testing Market#Global Hematology Testing Market Report#Global Hematology Testing Market Industry
0 notes
Text
Key Players in the POC Analyzers Market: A Company Spotlight
The Point-of-Care (POC) Analyzers Market is vibrant, with several key players contributing to the development and advancement of portable diagnostic solutions.
Buy the Full Report for Detailed Segments Insights into the POC Analyzers Market, Download a Free Report Sample
This company spotlight highlights some of the prominent players in this dynamic market:
Abbott Laboratories:
Abbott is a global healthcare company with a diverse portfolio, including a range of POC analyzers. Their offerings cover areas such as blood glucose monitoring, cardiac markers, infectious diseases, and molecular diagnostics. Abbott aims to provide rapid and accurate diagnostic solutions to improve patient outcomes.
Roche Diagnostics:
Roche Diagnostics, a division of the Swiss multinational Roche Group, is a leading player in the POC analyzers market. Their portfolio includes devices for testing blood gases, electrolytes, cardiac markers, and coagulation parameters. Roche focuses on delivering reliable and efficient solutions for decentralized testing.
Siemens Healthineers:
Siemens Healthineers is a global medical technology company known for its innovative diagnostic solutions. In the POC analyzers market, Siemens offers devices for testing blood gases, cardiac markers, and infectious diseases. Their emphasis on connectivity and data management contributes to streamlined healthcare workflows.
Danaher Corporation (Beckman Coulter):
Beckman Coulter, a subsidiary of Danaher Corporation, is a key player in the POC diagnostics space. Their POC analyzers cover a wide range of parameters, including chemistry, hematology, and immunoassays. Beckman Coulter focuses on delivering solutions that enhance clinical decision-making.
Sysmex Corporation:
Sysmex, a Japanese multinational corporation, is a prominent player in the global in-vitro diagnostics market. In the POC analyzers segment, Sysmex offers solutions for hematology testing, contributing to rapid and accurate blood analysis in various healthcare settings.
Quidel Corporation:
Quidel Corporation specializes in diagnostic testing solutions, including a range of POC analyzers. Their portfolio covers tests for infectious diseases, cardiac markers, and women's health. Quidel aims to provide rapid and accurate results to facilitate timely clinical decisions.
Nova Biomedical:
Nova Biomedical is a U.S.-based company known for its commitment to POC testing solutions. They offer analyzers for blood gas, electrolyte, and critical care testing. Nova Biomedical focuses on delivering high-performance POC analyzers for use in various healthcare settings.
Alere Inc. (Now part of Abbott):
Alere, acquired by Abbott, was a major player in POC diagnostics before the acquisition. Their portfolio included rapid tests and analyzers for infectious diseases, cardiac markers, and women's health. The integration with Abbott has strengthened the company's position in the POC market.
BioMérieux SA:
BioMérieux, a French multinational company, is a key player in the field of in-vitro diagnostics. In the POC analyzers market, BioMérieux focuses on solutions for infectious disease testing, including molecular diagnostics and immunoassays.
PTS Diagnostics:
PTS Diagnostics specializes in developing and manufacturing POC diagnostic products. Their portfolio includes analyzers for lipid panels, diabetes management, and cardiovascular risk assessment. PTS Diagnostics aims to provide cost-effective and user-friendly solutions for decentralized testing.
It's important to note that the POC analyzers market is dynamic, and the landscape may have evolved since my last knowledge update. Additionally, new players may have entered the market, and strategic partnerships or acquisitions may have occurred. For the latest information, it's advisable to refer to recent reports, company announcements, and industry publications.
0 notes
Text
Hemostasis/ Coagulation Analyzer Market is driven by increasing prevalence of chronic and lifestyle diseases

The hemostasis and coagulation analyzer market primarily includes point-of-care hemostasis testing analyzers and clinical laboratory hemostasis testing analyzers that are used to test various blood coagulation parameters and processes for diagnostic and treatment monitoring purposes. These analyzers measure parameters like coagulation factors, fibrinogen levels, D-dimer and prothrombin time and help detect bleeding disorders, determine therapy, and monitor anticoagulant drug therapy among others. The advantages of these analyzers are that they provide fast and automated testing methods for hemostasis and coagulation parameters as compared to traditional manual methods. Considering the rising lifestyle and chronic diseases like cardiovascular diseases and cancer which require coagulation monitoring, there is a growing need for these analyzers in hospitals, clinics and diagnostic centers.
The Global Hemostasis/ Coagulation Analyzer Market is estimated to be valued at US$ 6.47 Bn in 2024 and is expected to exhibit a CAGR of 5.8% over the forecast period 2024 to 2031.
Key Takeaways
Key players operating in the Hemostasis/ Coagulation Analyzer Market Growth are Horiba Medical, Siemens Healthineers AG, Agappe Diagnostics Ltd, NIHON KOHDEN CORPORATION, Sysmex Corporation, F. Hoffmann-La Roche Ltd, Stago Group, Maccura Biotechnology Co., Ltd., Thermo Fisher Scientific Inc., Haemonetics Corporation, Genrui Biotech Co., Ltd., BIOLABO S.A.S, Erba Mannheim, Sclavo Diagnostics International, and Helena Laboratories Corporation. Second paragraph is talking about the growing demand in market and third paragraph is talking about global expansion of market.
The global hemostasis and coagulation analyzer market is expected to witness significant growth over the forecast period driven by the steadily increasing global incidence of chronic diseases such as cardiovascular diseases, cancer, diabetes as well as rising demand for point-of-care testing. Coagulation disorders occur frequently in cancer, and monitoring of coagulation parameters is vital during cancer diagnosis and treatment which will boost the demand for these analyzers. Furthermore, technological advancements leading to the development of advanced rapid point-of-care testing analyzers will augment the adoption across healthcare settings.
Geographically, North America is anticipated to dominate the global market over the forecast years owing to increasing healthcare expenditure, availability of advanced hemostasis testing products, and presence of leading market players in the region. However, the Asia Pacific region is projected to exhibit the highest growth rate owing to improving access to diagnostics, increasing healthcare spending, rising patient pool of chronic diseases in counties like China and India. Product launches and expansions into emerging markets will further aid in the global expansion of key hemostasis and coagulation analyzer companies.
Get More Insights On This Topic: Hemostasis/Coagulation Analyzer Market
#Hemostasis#Coagulation Analyzer Market Growth#Coagulation Analyzer Market Size#Coagulation Analyzer Market Share#Coagulation Analyzer#Coagulation Analyzer Market Scope#Clinical Laboratory Analyzers#Reagents
0 notes
Text
Hemostasis/Coagulation Analyzer Market Top Winning Strategies and Industry Forecast 2023-2032
The field of hemostasis and coagulation diagnostics is undergoing a transformative phase, driven by rapid advancements in analyzer technology. This article delves into the current market insights, explores the revolutionary technologies shaping hemostasis analyzers, and provides a glimpse into the promising future prospects of this dynamic industry.
𝐑𝐞𝐪𝐮𝐞𝐬𝐭 𝐒𝐚𝐦𝐩𝐥𝐞 𝐂𝐨𝐩𝐲 𝐨𝐟 𝐑𝐞𝐩𝐨𝐫𝐭 : https://www.alliedmarketresearch.com/request-toc-and-sample/8012
Current Market Landscape:
The hemostasis/coagulation analyzer market is experiencing notable growth, fueled by an increasing prevalence of bleeding and clotting disorders worldwide. Diagnostic advancements are crucial in managing these conditions effectively, and the demand for more accurate, efficient, and rapid diagnostic tools has never been higher. The market is witnessing a surge in research and development activities, with a focus on delivering cutting-edge technologies that redefine the standards of hemostasis testing.
Revolutionary Technologies:
Point-of-Care Testing (POCT): The integration of point-of-care testing in hemostasis analysis is a game-changer. Portable analyzers that deliver rapid results at the patient's bedside are gaining traction, offering immediate insights for critical decision-making in emergency situations.
Automation and Robotics: Automation is streamlining hemostasis testing processes, reducing manual errors, and enhancing overall efficiency. Robotic systems are being employed to handle sample processing, allowing for increased throughput and consistency in results.
Enhanced Connectivity Solutions: The era of connectivity is influencing hemostasis analyzers, with the integration of cloud-based technologies and data analytics. This connectivity not only facilitates real-time monitoring but also opens avenues for telemedicine and remote diagnostics.
Multiplex Testing: Advancements in multiplexing technologies enable the simultaneous analysis of multiple coagulation parameters from a single sample. This not only saves time but also provides a comprehensive overview of a patient's coagulation profile.
Market Insights:
The COVID-19 pandemic has underscored the importance of hemostasis analyzers in managing critical conditions. The market has witnessed a notable uptick in demand for these analyzers, with a particular focus on their role in understanding the coagulation abnormalities associated with severe cases of the virus. This increased awareness and usage during the pandemic have positioned hemostasis analyzers as indispensable tools in modern healthcare.
𝐏𝐫𝐞-𝐛𝐨𝐨𝐤 𝐭𝐡𝐢𝐬 𝐑𝐞𝐩𝐨𝐫𝐭 𝐍𝐨𝐰 : https://www.alliedmarketresearch.com/hemostasis-coagulation-analyzer-market/purchase-options
Sysmex Corporation's Next-Gen Haemostasis Analyzers: Elevating Efficiency and Operability
Cutting-Edge Automation with CN-6000 and CN-3000: In December 2018, Sysmex Corporation made waves in the haemostasis industry with the launch of its next-generation automated blood coagulation analyzers, CN-6000 and CN-3000. These analyzers represent a significant leap forward in blood clotting technology, setting new standards for efficiency and operability. By introducing these advanced systems, Sysmex aims to cater to diverse customer demands effectively while substantially increasing operational efficiency in the haemostasis sector.
Sysmex CS-2500 System by Siemens Healthineers: Revolutionizing Mid-Volume Labs: In April 2018, Siemens Healthineers Laboratory Diagnostics unveiled the Sysmex CS-2500 System, a revolutionary addition to mid-volume labs. This cutting-edge system integrates proven PSI technologies, providing mid-volume labs with the capability to standardize test outcomes. The Sysmex CS-2500 not only brings advanced hemostasis technologies to local comparison labs but also seamlessly integrates into embedded distribution networks (IDNs). This launch marks a strategic move to enhance the accessibility and standardization of hemostasis testing, empowering laboratories with advanced capabilities for efficient and reliable diagnostics.
Meeting Varied Customer Demands and Increasing Industry Efficiency: Both product launches by Sysmex Corporation underscore a commitment to meeting diverse customer demands and driving efficiency in the haemostasis industry. The introduction of the CN-6000, CN-3000, and Sysmex CS-2500 represents a concerted effort to advance automation, improve operational workflows, and elevate the overall standards of blood coagulation analysis. As Sysmex continues to pioneer innovation in the field, these launches position the company as a frontrunner in shaping the future of haemostasis diagnostics, with a focus on precision, reliability, and enhanced customer satisfaction.
Future Prospects:
Looking ahead, the hemostasis/coagulation analyzer market is poised for significant growth. The integration of artificial intelligence (AI) and machine learning (ML) is anticipated to revolutionize result interpretation and enhance predictive analytics. Personalized medicine approaches, tailored to an individual's coagulation profile, are expected to become more prevalent, paving the way for more targeted and effective treatment strategies.
Strategic collaborations between diagnostic companies and research institutions are likely to accelerate innovation, bringing forth novel technologies that push the boundaries of hemostasis diagnostics. As the industry continues to evolve, accessibility to advanced hemostasis analyzers, especially in resource-limited settings, will be a key focus, ensuring that the benefits of these technological advancements reach diverse populations.
𝐈𝐧𝐭𝐞𝐫𝐞𝐬𝐭𝐞𝐝 𝐭𝐨 𝐏𝐫𝐨𝐜𝐮𝐫𝐞 𝐭𝐡𝐞 𝐑𝐞𝐬𝐞𝐚𝐫𝐜𝐡 𝐑𝐞𝐩𝐨𝐫𝐭? 𝐈𝐧𝐪𝐮𝐢𝐫𝐞 𝐁𝐞𝐟𝐨𝐫𝐞 𝐁𝐮𝐲𝐢𝐧𝐠 : https://www.alliedmarketresearch.com/purchase-enquiry/8012
Conclusion:
The advancements in hemostasis/coagulation analyzer technology are reshaping the landscape of diagnostic medicine. With a blend of innovative technologies, connectivity solutions, and a commitment to addressing emerging healthcare challenges, the future of hemostasis diagnostics holds promise for more precise, efficient, and patient-centric care. The journey towards these advancements signifies a transformative era in the diagnosis and management of coagulation disorders.
About Us
Allied Market Research (AMR) is a full-service market research and business-consulting wing of Allied Analytics LLP based in Wilmington, Delaware. Allied Market Research provides global enterprises as well as medium and small businesses with unmatched quality of "Market Research Reports" and "Business Intelligence Solutions." AMR has a targeted view to provide business insights and consulting to assist its clients to make strategic business decisions and achieve sustainable growth in their respective market domain.
Pawan Kumar, the CEO of Allied Market Research, is leading the organization toward providing high-quality data and insights. We are in professional corporate relations with various companies and this helps us in digging out market data that helps us generate accurate research data tables and confirms utmost accuracy in our market forecasting. Each and every data presented in the reports published by us is extracted through primary interviews with top officials from leading companies of domain concerned. Our secondary data procurement methodology includes deep online and offline research and discussion with knowledgeable professionals and analysts in the industry.
0 notes
Text
Global Antivenom Market Overview – Market Growth Analysis And Key Drivers
The Antivenom Global Market Report 2023, provides comprehensive information on the antivenom market across 60+ geographies in the seven regions - Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa for the 27 major global industries. The report covers a ten year historic period – 2010-2021, and a ten year forecast period – 2023-2032.
Learn More On The Antivenom Market’s Growth:
The global antivenom market size is expected to grow from $1.66 billion in 2022 to $1.80 billion in 2023 at a compound annual growth rate (CAGR) of 8.2%. The Russia-Ukraine war disrupted the chances of global economic recovery from the COVID-19 pandemic, at least in the short term. The war between these two countries has led to economic sanctions on multiple countries, a surge in commodity prices, and supply chain disruptions, causing inflation across goods and services and affecting many markets across the globe. The antivenom market size is expected to reach $2.37 billion in 2027 at a CAGR of 7.1%.
Get A Free Sample Of The Report (Includes Graphs And Tables):
Product innovations are a key trend gaining popularity in the antivenom market. Companies operating in the antivenom market are developing innovative products to sustain their position in the market. For instance, in April 2021, Rare Disease Therapeutics, Inc., a US-based pharmaceutical company, launched Antivenom ANAVIP®, a new expanded FDA-approved indication. This medication is licensed to treat pit viper bites in adults and children, including rattlesnakes, copperheads, and cottonmouth/water moccasins. It was created with a lengthy half-life to lessen the possibility of re-emergent venom effects (such as platelet drop, longer bleeding times, and other abnormal blood coagulation tests), which commonly necessitated additional doses of a shorter-acting antivenom.
The antivenom market is segmented:
1) By Type: Monovalent, Polyvalent, Other Types
2) By Animal: Snakes, Scorpions, Spiders, Other Animals
3) By Mode of Action: Cytotoxic, Neurotoxic, Haemotoxic, Cardiotoxic, Myotoxic, Other Modes Of Action
4) By End User: Hospitals, Clinics, Ambulatory Surgical Centers, Other End-Users
North America was the largest region in the antivenom market in 2022.
The table of contents in TBRC’s antivenom market report includes:
1. Executive Summary
2. Market Characteristics
3. Market Trends And Strategies
4. Impact Of COVID-19
5. Market Size And Growth
6. Segmentation
7. Regional And Country Analysis
.
.
.
27. Competitive Landscape And Company Profiles
28. Key Mergers And Acquisitions
29. Future Outlook and Potential Analysis
Learn About Us:
The Business Research Company is a market intelligence firm that pioneers in market, company, and consumer research. TBRC’s specialist consultants are located globally and are experts in a wide range of industries that include healthcare, manufacturing, financial services, chemicals, and technology. The firm has offices located in the UK, the US, and India, along with a network of proficient researchers in 28 countries. Through the report businesses can gain a thorough understanding of the market’s size, growth rate, major drivers and leading players.
Contact Us:
The Business Research Company
Europe: +44 207 1930 708
Asia: +91 88972 63534
Americas: +1 315 623 0293
Email: [email protected]
Follow Us On:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
Twitter: https://twitter.com/tbrc_info
Facebook: https://www.facebook.com/TheBusinessResearchCompany
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Blog: https://blog.tbrc.info/
Healthcare Blog: https://healthcareresearchreports.com/
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
0 notes
Text
Emerging Technologies Driving the Blood Collection Tubes Market by 2030

The global blood collection tubes market is expected to grow US$ 3.20 billion by 2030, at a CAGR of 3.02%. The growth of the market is attributed to the increasing demand for blood diagnostic tests, growing prevalence of chronic diseases, and rising geriatric population.
Blood collection tubes are essential tools for collecting blood samples for diagnostic testing and other medical procedures. They are used to collect a variety of blood samples, including whole blood, serum, plasma, and blood gas. Blood collection tubes are available in a variety of materials, including glass, plastic, and PET.
Request A Sample Copy of This Research Report! https://absolutemarketresearch.com/Global-Blood-Collection-Tubes-Market/1977/request-sample
Samples of patient blood, urine, and serum are collected in sterilized glass or plastic blood collection tubes in order to diagnose blood-related illnesses. Blood collection tubes come in a variety of forms, such as sodium heparin tubes, vacutainer blood collection tubes, plasma separation tubes, serum separating tubes, and EDTA tubes.
In order to diagnose blood-related disorders, patient blood, urine, and serum samples are collected using sterilized plastic or glass blood collection tubes. Vacutainer blood collection tubes, plasma separation tubes, serum separating tubes, EDTA tubes, and sodium heparin tubes are only a few of the several varieties of blood collection tubes available.
Key Takeaways:
The global blood collection tubes market is expected to grow at a CAGR of 3.02% from 2023 to 2030, reaching a value of US$ 3.20 billion by 2030.
The increasing prevalence of chronic diseases, rising demand for point-of-care testing, and growing geriatric population are key drivers of the market growth.
The serum separation tubes segment accounted for the largest market share in 2022 and is expected to continue to dominate the market during the forecast period.
North America is expected to remain the largest market for blood collection tubes throughout the forecast period, due to the high prevalence of chronic diseases and advanced healthcare infrastructure in the region.
Asia Pacific is expected to be the fastest-growing market during the forecast period, due to the increasing prevalence of chronic diseases, rising demand for point-of-care testing, and growing geriatric population in the region.
Regional Outlook:
The global blood collection tubes market is segmented into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. North America is the largest market for blood collection tubes, followed by Europe and Asia Pacific. Asia Pacific is expected to be the fastest-growing market during the forecast period, due to the increasing prevalence of chronic diseases, rising demand for point-of-care testing, and growing geriatric population in the region.
Key Players:
The key players in the global blood collection tubes market include:
BD
Terumo
Greiner Bio-One
Sarstedt
Vacutainer Systems
Jiangsu Yuyue Medical Equipment & Supplies Group Co., Ltd.
Nipro Corporation
Jiangsu Kanghui Medical Equipment Co., Ltd.
Zhejiang Kangda Medical Equipment Co., Ltd.
Hangzhou Singclean Medical Products Co., Ltd.
Sichuan Haimen Medical Equipment Co., Ltd.
Segmentation:
The global blood collection tubes market is segmented by type, material, and application.
By type, the market is segmented into:
Serum separation tubes
Coagulation tubes
Plasma collection tubes
Blood bank tubes
Microbiology tubes
Others
By material, the market is segmented into:
Plastic
Glass
Others
By application, the market is segmented into:
Hospitals
Diagnostic laboratories
Blood banks
Others
0 notes