#moderna inc
Text
Dr. Peter McCullough : Les publications montrent clairement que c'est une opération gouvernementale qui a créé le SRAS-CoV-2. (2 minutes, 55 secondes)


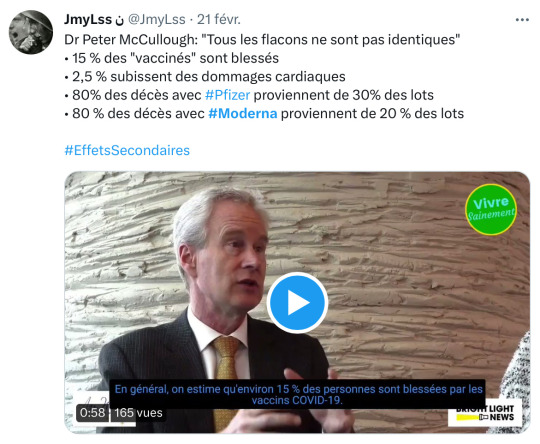
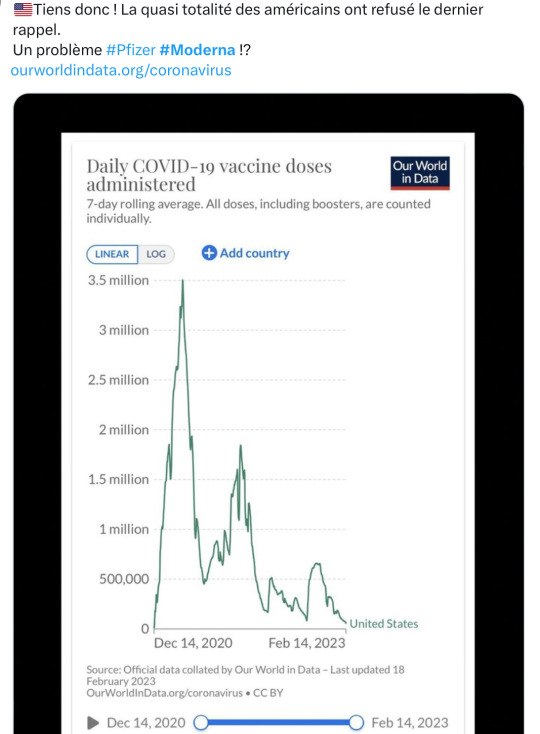

Le Bancel dont il est question dans la vidéo avec McCullough est le boss de la nouvelle fabrique de piquouzes que va nous imposer le dynamique cabinet de McKinsey, Trudeau & Legault, les influshables.
#big pharma est toujours en bonne santé#covid&corruption#covid de sens#covid au cerveau#bas les masques#big pharmla#mon infirmier est un fumier#mon médecin me rend malade#mon ministre est sinistre#bancel stephane#moderna inc#vaccin corrompu#vaccinations#vaccination des enfants#pas grave car big pharma va nous trouver un vaccin ou bedon une pinune#covid
16 notes
·
View notes
Text
U.S. Senator Bernie Sanders sent Moderna Inc (MRNA.O) a letter this week asking the drug company to halt planned U.S. price increases on its COVID-19 vaccine, saying price hikes could make the shot unaffordable for millions of Americans.
Sanders said in his letter that raising prices would be particularly egregious after the U.S. government provided around $1.7 billion to fund development of the vaccine. The letter was addressed to Moderna Chief Executive Stephane Bancel.
"You propose to make the vaccine unaffordable for the residents of this country who made the production of the vaccine possible," wrote Sanders, who is set to become chairman of the Senate's Health, Education, Labor and Pensions later this month. "That is not acceptable."
Moderna has not settled on a price yet, but Bancel has said that a range of $110 to $130 a dose for the vaccine once the United States moves to a commercial market for the shots is reasonable given the value they create.
The top end of that range is around eight times the price in the earliest U.S. contracts for the vaccine and nearly five times the roughly $27 a dose the government paid for booster shots last year.
"While we are still in discussions with stakeholders on the price of our COVID-19 vaccines, Moderna is committed to pricing that reflects the value that COVID-19 vaccines bring to patients, healthcare systems, and society," Moderna said in an emailed statement.
The company said that under the Affordable Care Act, its COVID-19 shots will continue to be available at no cost for most Americans.
Sanders is a democratic socialist whose presidential campaigns have pushed the U.S. Democratic Party agenda leftward. The Vermont Senator has railed against high drug prices and backed Medicare-for-all, and his chairmanship of the HELP committee could put drug companies in his crosshairs.
Sanders wrote that Bancel and several of Moderna's founders have become billionaires after the vaccine's launch. He said the higher prices would cost U.S. taxpayers billions of dollars and make the shots too expensive for uninsured and underinsured Americans.
"Now, in the midst of a continuing public health crisis and a growing federal deficit, is not the time for Moderna to be quadrupling the price of this vaccine. Now is not the time for unacceptable corporate greed," Sanders wrote.
Moderna's COVID-19 vaccine sales were around $18.4 billion in 2022. That is expected to fall sharply next year even with the price increases, as demand for the shots has dropped off. The drugmaker said on Monday it expects a minimum of $5 billion in revenue from the shots this year.
#us politics#news#reuters#sen. bernie sanders#vermont#2023#Moderna Inc#Stephane Bancel#senate health education labor and pensions committee#health education labor and pensions committee#us senate#bernie for the people#bernie gets it#bernie forever#Affordable Care Act#affordable healthcare#covid vaccine#coronavirus vaccine#corporate greed#us healthcare#for profit healthcare
8 notes
·
View notes
Text

In my head I was all “ohh, alien lookin”
1 note
·
View note
Text
Stocks rise as investors look ahead to key consumer inflation data
Stocks rise as investors look ahead to key consumer inflation data
Stocks rose Wednesday as investors shook off inflation data that came in higher than expected and looked ahead to a key consumer report that will inform the pace of the Federal Reserve’s rate hikes going forward.
The Dow Jones Industrial Average gained 169 points, or 0.58%. The S&P 500 rose 0.28%, bolstered by a 10% jump in shares of Moderna, the top gaining stock in the index. The Nasdaq…

View On WordPress
#Breaking News: Markets#business news#Economic events#Investment strategy#Jerome Powell#Markets#Moderna Inc#Prices#Rocket Lab USA Inc#Stock markets#United States
1 note
·
View note
Text
EU regulator clears tweaked versions of COVID vaccines
EU regulator clears tweaked versions of COVID vaccines
2022-09-04 08:02:48
LONDON (AP) — The European Medicines Agency has recommended the authorization of two coronavirus vaccines made by Pfizer-BioNTech and Moderna Inc., tweaked to include protection against an early version of the omicron variant.
In a statement on Thursday, the EU drug regulator said the two messenger RNA boosters offered protection both against the original version of COVID-19…
View On WordPress
0 notes
Text
FDA authorizes emergency use for Novavax Covid-19 vaccine for ages 12 to 17
FDA authorizes emergency use for Novavax Covid-19 vaccine for ages 12 to 17
A box of the Novavax Covid-19 vaccine arranged at a pharmacy in Schwenksville, Pennsylvania, US, on Monday, Aug. 1, 2022.
Bloomberg | Bloomberg | Getty Images
Biotechnology company Novavax announced on Friday that its Covid-19 vaccine has been authorized for emergency use by the U.S Food and Drug Administration for adolescents between the ages of 12 and 17.
In July, Novavax’s two-dose Covid-19…
View On WordPress
#ages#authorizes#Biotech and Pharmaceuticals#business#business news#Coronavirus#Coronavirus: Prevention#COVID19#emergency#FDA#Health care industry#Moderna Inc#Novavax#Novavax Inc#Pfizer Inc#Science#vaccine
0 notes
Text
Ouest-France: ENTRETIEN. Covid-19 : « Le vaccin de Moderna sera plus efficace contre les variants »
Ouest-France: ENTRETIEN. Covid-19 : « Le vaccin de Moderna sera plus efficace contre les variants ».
https://www.ouest-france.fr/sante/vaccin/entretien-covid-19-le-vaccin-de-moderna-sera-plus-efficace-contre-les-variants-5b2cb650-f7c2-11ec-91dc-9e5b156d5995
0 notes
Text
Vaccines that protect against severe illness, death and lingering long Covid-19 symptoms from a coronavirus infection were linked to small increases in neurological, blood, and heart-related conditions in the largest global vaccine safety study to date.
The rare events – identified early in the pandemic – included a higher risk of heart-related inflammation from mRNA shots made by Pfizer Inc, BioNTech SE, and Moderna Inc, and an increased risk of a type of blood clot in the brain after immunisation with viral-vector vaccines such as the one developed by the University of Oxford and made by AstraZeneca Plc.
The viral-vector jabs were also tied to an increased risk of Guillain-Barre syndrome, a neurological disorder in which the immune system mistakenly attacks the peripheral nervous system.
More than 13.5 billion doses of Covid vaccines have been administered globally over the past three years, saving over 1 million lives in Europe alone. Still, a small proportion of people immunised were injured by the shots, stoking debate about their benefits versus harms.
The new research, by the Global Vaccine Data Network, was published in the journal Vaccine last week.
The research looked for 13 medical conditions that the group considered “adverse events of special interest” among 99 million vaccinated individuals in eight countries, aiming to identify higher-than-expected cases after a Covid shot.
Myocarditis, or inflammation of the heart muscle, was consistently identified following a first, second and third dose of mRNA vaccines, the study found.
The highest increase in the observed-to-expected ratio was seen after a second jab with the Moderna shot. A first and fourth dose of the same vaccine was also tied to an increase in pericarditis, or inflammation of the thin sac covering the heart.
Researchers found a statistically significant increase in cases of Guillain-Barre syndrome within 42 days of an initial Oxford-developed ChAdOx1 or “Vaxzevria” shot that wasn’t observed with mRNA vaccines.
Based on the background incidence of the condition, 66 cases were expected – but 190 events were observed.
ChAdOx1 was linked to a threefold increase in cerebral venous sinus thrombosis, a type of blood clot in the brain, identified in 69 events, compared with an expected 21.
The small risk led to the vaccine’s withdrawal or restriction in Denmark and multiple other countries. Myocarditis was also linked to a third dose of ChAdOx1 in some, but not all, populations studied.
Possible safety signals for transverse myelitis – spinal cord inflammation – after viral-vector vaccines was identified in the study.
So was acute disseminated encephalomyelitis – inflammation and swelling in the brain and spinal cord – after both viral-vector and mRNA vaccines.
Seven cases of acute disseminated encephalomyelitis after vaccination with the Pfizer-BioNTech vaccine were observed, versus an expectation of two.
The adverse events of special interest were selected based on pre-established associations with immunisation, what was already known about immune-related conditions and preclinical research. The study didn’t monitor for postural orthostatic tachycardia syndrome, or POTS, that some research has linked with Covid vaccines.
Exercise intolerance, excessive fatigue, numbness and “brain fog” were among common symptoms identified in more than 240 adults experiencing chronic post-vaccination syndrome in a separate study conducted by the Yale School of Medicine. The cause of the syndrome isn’t yet known, and it has no diagnostic tests or proven remedies.
The Yale research aims to understand the condition to relieve the suffering of those affected and improve the safety of vaccines, said Harlan Krumholz, a principal investigator of the study, and director of the Yale New Haven Hospital Centre for Outcomes Research and Evaluation.
“Both things can be true,” Krumholz said in an interview. “They can save millions of lives, and there can be a small number of people who’ve been adversely affected.”
31 notes
·
View notes
Text
5 things to know before the stock market opens Thursday, September 1
5 things to know before the stock market opens Thursday, September 1
2022-09-01 04:22:00
Cleveland Federal Reserve President and CEO Loretta Mester gives her keynote address at the 2014 Financial Stability Conference in Washington December 5, 2014.
Gary Cameron | Reuters
Here are the most important news items that investors need to start their trading day:
1. Stocks can’t shake it off
So much for a fresh start in September. U.S. equities markets were primed…
View On WordPress
#Business#business news#Economy#Investment strategy#Joe Biden#markets#Moderna Inc#NVIDIA Corp#Pfizer Inc#Ukraine#Vladimir Putin#Walmart Inc
0 notes
Text
FDA authorizes emergency use for Novavax Covid-19 vaccine for ages 12 to 17
FDA authorizes emergency use for Novavax Covid-19 vaccine for ages 12 to 17
A box of the Novavax Covid-19 vaccine arranged at a pharmacy in Schwenksville, Pennsylvania, US, on Monday, Aug. 1, 2022.
Bloomberg | Bloomberg | Getty Images
Biotechnology company Novavax announced on Friday that its Covid-19 vaccine has been authorized for emergency use by the U.S Food and Drug Administration for adolescents between the ages of 12 and 17.
In July, Novavax’s two-dose Covid-19…
View On WordPress
#ages#authorizes#Biotech and Pharmaceuticals#business#business news#Coronavirus#Coronavirus: Prevention#COVID19#emergency#FDA#Health care industry#Moderna Inc#Novavax#Novavax Inc#Pfizer Inc#Science#vaccine
0 notes