#antineoplastic
Text
Amcenestrant (SAR 439859)
Amcenestrant (SAR 439859)
アムセネストラント
Molecular Weight554.48FormulaC31H30Cl2FNO3CAS No.2114339-57-8
6-(2,4-dichlorophenyl)-5-[4-[(3S)-1-(3-fluoropropyl)pyrrolidin-3-yl]oxyphenyl]-8,9-dihydro-7H-benzo[7]annulene-2-carboxylic acid
Amcenestrant
8-(2,4-dichlorophenyl)-9-(4-{[(3 S )-1-(3-fluoropropyl)pyrrolidin-3-yl]oxy}phenyl)-6,7-dihydro – 5H- Benzo[7]annulene-3-carboxylic…
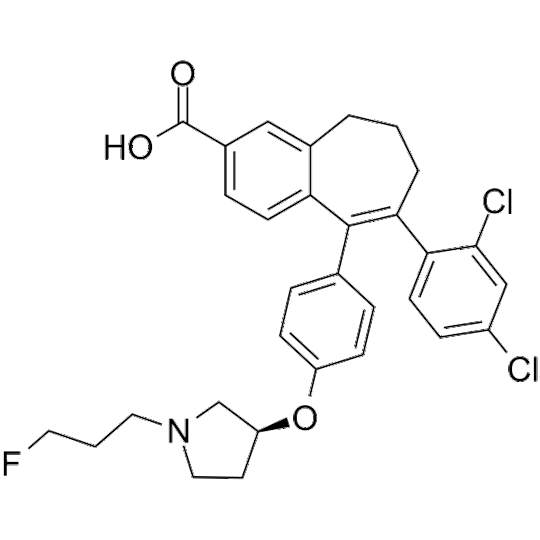
View On WordPress
0 notes
Text
https://carbonfacesocial.org/blogs/46708/Antineoplastic-Drugs-Market-Overview-Size-Share-and-Forecast-2031
The Antineoplastic Drugs Market in 2023 is US$ 134.66 billion, and is expected to reach US$ 338.70 billion by 2031 at a CAGR of 12.22%.
#Antineoplastic Drugs Market#Antineoplastic Drugs Market Trends#Antineoplastic Drugs Market Research
0 notes
Text
Dosage, adverse reactions, and contraindications of pirarubicin
Pirarubicinis an anthracycline cell cycle non-specific antineoplastic drug synthesized from doxorubicin and dihydropyran. It is suitable for the treatment of breast cancer. It is also effective in patients with drug resistance.
The mechanism of action of the drug is that it can directly intercalate between DNA double strands, inhibit DNA polymerase, prevent nucleic acid synthesis, and prevent cells from dividing in the G2 phase, resulting in tumor cell death.
Dosage
Dissolve this product in 10ml of 5% glucose injection or water for injection. Intravenous, arterial, and intravesical infusion.
Intravenous administration: generally 25-40 mg/m2 per body surface area. For breast cancer, the recommended combination is 40-50 mg/m2 each time. It is administered on the first day of each course of treatment and can be repeated at 21-day intervals according to the patient's blood picture. For acute leukemia, the adult dose is 25 mg/m2 once based on body surface area.
Arterial administration: For head and neck cancer, 7-20 mg/m2 per body surface area, once a day, for 5-7 days, or 14-25 mg/m2 each time, once a week.
Intravesical administration: for the prevention of postoperative recurrence of superficial bladder cancer. According to the body surface area, 15~30mg/m2 once, diluted to 500~1000μg/ml concentration, injected into the bladder cavity for 0.5h, once a week, 4~8 times in a row; then once a month, a total of 1 year.
Doctors can adjust the time, dosage, and frequency of administration according to the patient's condition.
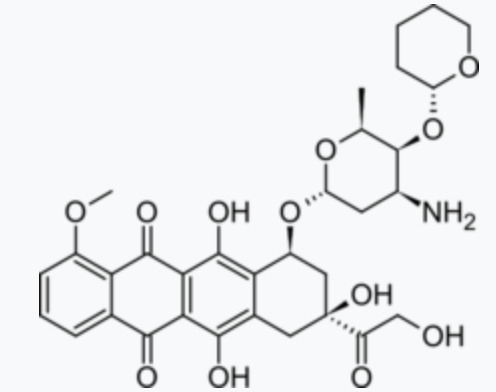
Adverse reactions
1. Myelosuppression is dose-limiting toxicity, mainly neutropenia, with an average minimum value of 14 days and recovery on the 21st day, anemia and thrombocytopenia are rare;
2. Cardiotoxicity is lower than doxorubicin, acute cardiotoxicity is mainly reversible ECG changes, such as arrhythmia or non-specific ST-T abnormalities, and chronic cardiotoxicity is dose-accumulating. The incidence of acute and chronic cardiotoxicity of this product is about 1/7 and 1/4 of doxorubicin.
3. Hair loss: The overall incidence of hair loss with this product is about 40%, which is significantly lower than that of doxorubicin (80%); the incidence of severe hair loss is about 20%, which is significantly lower than that of doxorubicin (60%).
4. Gastrointestinal reactions: nausea, vomiting, loss of appetite, oral mucositis, and sometimes diarrhea;
5. Others: abnormal liver and kidney function, skin pigmentation, etc., occasional rash. The intravesical injection can cause bladder irritation symptoms such as frequent urination, dysuria, occasional hematuria, and rarely bladder atrophy.
Taboo
1. Patients with obvious bone marrow suppression due to chemotherapy or radiotherapy are contraindicated;
2. Patients with severe structural heart disease or abnormal cardiac function and those who are allergic to this product are prohibited;
3. Patients who have used high-dose anthracyclines (such as doxorubicin or daunorubicin) are contraindicated;
4. Women of pregnancy, lactation, and childbearing age are prohibited.
Minbiotech is a Sirolimus supplier and factory, Minbiotech CAS 53123-88-9 sale, Sirolimus supplier, Welcome to visit our website.
Related news of Minbiotech bio-pharmacy
Where to buy sirolimus cheap
Introduction to Biosimilars
0 notes
Text
some background information
What am I doing? Why, I'm re-watching House, M.D. as a whole-ass pharmacist and taking meticulous notes!
I’m only going to focus on when House, M.D. gets something about medication just wildly wrong. I’m not going to be like “why are the chest compressions so slow” or “would Chase really be inserting that temporary transcutaneous pacer at the beside wouldn’t they want to do that in the EP lab” or anything else along those lines. I am but a humble pharmacist.
Hydrocodone/acetaminophen (APAP), the U.S. Food and Drug Administration (FDA), and the Drug Enforcement Administration (DEA)
This is going to be interesting because House aired from November 16, 2004 to May 21, 2012 and there are two things that come to mind for me: 1) The U.S. Food and Drug Administration (FDA) asked drug manufacturers to reduce the strength of acetaminophen in combination acetaminophen products to 325 mg in 2011(1) and 2) hydrocodone combination products were still scheduled as schedule III by the Drug Enforcement Administration (DEA).(2) Hydrocodone combination products weren’t rescheduled to schedule II until 2014. Schedule III prescriptions are a lot easier to write and fill than schedule II prescriptions. A lot of the legal nuance varies by state and I am not going to read New Jersey pharmacy statutes, thank you very MUCH I barely passed every Multistate Pharmacy Jurisprudence Examination (MPJE) I’ve ever taken. Back to the Vicodin. So how am I going to know exactly what formulation of Vicodin House was consuming?
I really don’t care about the hydrocodone component of it. There is no ceiling to an opioid, it’s whatever the patient can tolerate it. (Well technically there is too much to give but you have to look at if the patient is used to taking opioids or not don’t just slam someone with 10 mg of IV Dilaudid you know what I’m saying.) And if they don’t tolerate it give them some naloxone. And patients with chronic pain can tolerate a lot of opioids. Was Vicodin an appropriate opioid for House? No! But it was less regulated! And the threat of liver failure is dramatic! But Oxycontin would make more sense – Wilson’s an oncologist, he would be prescribing it all the time! Cancer hurts! Antineoplastics hurt! Oxycontin was the most widely abused prescription opioid in the United States in 2004(3), right when House started airing. But no, it’s Vicodin. The writers chose Vicodin for a reason. I’m going to let the art flow over me. We’re sticking with Vicodin. Or, hydrocodone/APAP because Abbott isn’t paying me to do this.
I worked in an independent retail pharmacy my first year of pharmacy school (which was 2010 before I realized I do not have a poker face to deal with the general public and immediately started working in a hospital), and I vaguely remember there being way too many formulations of hydrocodone/APAP. Hydrocodone/APAP has a few brand names: Vicodin, Norco, and Lortab. I usually say Norco because it’s the easiest. (I actually say hydrocodone/acetaminophen because I’m an asshole.) I found this email(4) sent by Abbott in October 2012 that discussed the newly reformulated Vicodin®, Vicodin ES®, and Vicodin HP®, which is handy since it lists the old formulations too:
Vicodin®
Hydrocodone 5 mg/acetaminophen 500 mg
Vicodin ES®
Hydrocodone 7.5 mg/acetaminophen 750 mg
Vicodin HP®
Hydrocodone 10 mg/acetaminophen 660 mg
Who the fuck puts 750 mg of acetaminophen in one tablet? That limits the patient to five tablets in 24 hours. We’ve known the maximum dose of acetaminophen is 4000 mg since the 1970s. What the fuck, Abbott.
(Matthew Mercer Voice) How Do You Want To Do This?
So, like, I guess I’ll just count the tablets I see House consuming and then calculate the total daily dose of acetaminophen for each Vicodin formulation? I feel like I can do total acetaminophen dosage based on episodes versus trying to keep track of the days. The timeline of this show is wonky and like once you hit the toxic dose you’re there so? Also, sometimes he takes more than one and it’s hard to hear the tablets like, clink against his teeth or whatever. Sparkle on! We’ll do our best. I was going to put this in an Excel spreadsheet but I just remembered I am a pharmacist and I cannot function in Excel.
Let’s talk about the mechanism of liver toxicity and treatment of acetaminophen overdose if we hit significant toxicity. Wouldn’t it be funny if we didn’t?? That would result in me rambling about House M.D. for pages and pages and he doesn’t even get hypothetical liver failure. Is this fanfiction? Am I writing really weird fanfiction? Anyways significant toxicity occurs if you hit 150 mg/kg of acetaminophen.(5) How much does Gregory House weigh?? Let’s give him a range: 160-180 pounds (73-82 kg). Fairly average, actually. So House’s toxic dose of acetaminophen is likely 10,950 mg to 12,300 mg. Let’s say lower range is 22 tablets of Vicodin®, 15 tablets of Vicodin ES®, 17 tablets of Vicodin HP® and the upper range is 25 tablets of Vicodin®, 17 tablets of Vicodin ES®, and 19 tablets of Vicodin HP®.
References
Department of Health and Human Services. Food and Drug Administration. Prescription drug products containing acetaminophen; actions to reduce liver injury from unintentional overdose. Federal Register. 2011;76(10):2691-2697.
Seago S, Hayek A, Pruszynski J, Newman MG. Change in prescription habits after federal rescheduling of hydrocodone combination products. Proc (Bayl Univ Med Cent). 2016; 29:268-270.
Zee AV. The promotion of marketing of OxyContin: commercial triumph, public health tragedy. Am J Public Health. 2009;99:221-227.
Abbott. Newly reformulated Vicodin® launch announcement. https://www.uspharmacist.com/email/ecf1248.html. Accessed January 8, 2024.
Hendrickson RG. Acetaminophen. In: Nelson LS, Howland M, Lewin NA, Smith SW, Goldfrank LR, Hoffman RS, eds. Goldfrank's Toxicologic Emergencies, 11e. McGraw-Hill; 2019:486-499.
#house md#hate crimes md#house rewatch 2024#I'm halfway through season 1 this is going to take A Bit#so please bear with me#but here's some background ramblings#I'll make a pinned post and have links to each season#so I am ~*~organized~*~
17 notes
·
View notes
Text
Pharma Manufacturing Company in India
OLGud Pharmaceutical is the best pharma manufacturing company in India. We export medicines according to the requirements of international buyers and export them worldwide. We offer 20+ categories including antidiabetics, antineoplastic, anti-TB, cardiac, gastrointestinal, gynaec, pain, respiratory, and more. Contact us today.

#Pharma manufacturing company in India#Pharma manufacturing company#best Pharma manufacturing company#Pharmaceutical Manufacturing company#OLGud Pharmaceutical
2 notes
·
View notes
Text
I decided to pop by good ol swanson to see what's cheap for supplements today and decided to look up two i'd never heard of before:
Pyrroloquinoline quinone [pubchem]
Apigenin [pubchem]
Astaxanthin [pubchem]
The former seems to be of an antioxidant nature similar to our friend emoxypine, and the latter is billed by pubchem as "antineoplastic" which apparently means it inhibits cancer cell development.
I'm ordering both, to see if there's any difference in antioxidant effects on my overall ability to function. Different interactions could mean different outcomes.
Apigenin is interesting for a studies relating to cellular regulation, especially when it comes to collagen and bone. Important for people who aren't spring chickens and have shitty joints.
Edit: adding 1 more.
#medical stuff#emoxypine#apigenin#pqq#pyrroloquinoline quinone#Astaxanthin#chemical names sound a lot like old atari titles imo
3 notes
·
View notes
Text
"Medicinal mushrooms have been proposed as a novel therapy that may improve cancer treatment and patients’ survival. They have been used medicinally since at least 3000 bce. Mushrooms are reported to have antimicrobial, anti-inflammatory, cardiovascular-protective, antidiabetic, hepatoprotective, and anticancer properties. It is well-established that mushrooms are adept at immune modulation and affect hematopoietic stem cells, lymphocytes, macrophages, T cells, dendritic cells (DCs), and natural killer (NK) cells.1 Extensive research over the last 40 years has demonstrated that mushrooms have potent antineoplastic properties that slow growth of tumors, regulate tumor genes, decrease tumoral angioneogenesis, and increase malignant-cell phagocytosis. Additionally, evidence suggests that medicinal mushrooms may safely boost chemotherapeutic efficacy and simultaneously protect against bone marrow suppression."
#mycology#medicinal mushrooms#mycelium#integrative medicine#herbal medicine#print this off later#fav
0 notes
Text
Poziotinib Researches on Cancers

Poziotinib has been investigated in clinical trials for the treatment of various cancers. Some of the specific conditions studied include:
Breast Cancer
Metastatic Breast Cancer
Increased Drug Resistance
Adenocarcinoma of Lung Stage IV
Adenocarcinoma of Lung Stage IIIB
These trials explore the effectiveness of Poziotinib in targeting cancers that overexpress or have mutations in the epidermal growth factor receptors (EGFR), including HER2 and HER4. Its use is particularly focused on cancers with EGFR exon 20 insertion mutations.
What Is Poziotinib Peptide?
Poziotinib peptide is an orally bioavailable, quinazoline-based, small-molecule, irreversible pan-epidermal growth factor receptor (EGFR or HER) inhibitor with potential antineoplastic activity. It inhibits EGFR (HER1 or ErbB1), HER2, HER4, and EGFR mutants, thereby hindering the proliferation of tumor cells that overexpress these receptors. EGFRs, which are cell surface receptor tyrosine kinases, are frequently upregulated in various cancer cell types and play crucial roles in cellular proliferation and survival.
Poziotinib has been investigated in clinical trials for the treatment of various cancers, including breast cancer, metastatic breast cancer, increased drug resistance, adenocarcinoma of the lung (Stage IV and Stage IIIB), among others.
For your safety, please buy and use Poziotinib with caution.
What Benefits Can You Get From Poziotinib on NSCLC?
The antitumor activity of poziotinib was found to be significant in patients with EGFR exon 20 positive non–small cell lung cancer (NSCLC). The efficacy of the treatment was found to be highly dependent on the location of the exon 20 loop insertion. These findings were published in Cancer Cell. The results were published in Cancer Cell.
The study, designated as phase 2 (NCT03066206), included 50 patients with advanced non-small cell lung cancer (NSCLC) who exhibited point mutations or insertions in the EGFR exon 20. The study achieved its primary end point with a confirmed objective response rate (ORR) of 32% (95% CI, 20.7%-45.8%) and 31% (95% CI, 19.1%-46%) by investigator and blinded independent review, respectively.
Moreover, researchers found a median progression-free survival (PFS) of 5.5 months (95% CI, 5.4-10.4) with a PFS rate of 43% (95% CI, 30%-60%) at 6 months, and 29% (95% CI, 18%-46%) at 12 months. Median overall survival also appeared to be beneficial at 19.2 months (95% CI, 11.8-24.1) with a disease control rate of 84% (95% CI, 71.5%-92%).
“EGFR exon 20 mutant lung cancers typically don’t respond well to the types of tyrosine kinase inhibitors that have been largely successful in targeting classical EGFR mutations, leaving this patient population with few effective treatment options,” said senior author of the study, John Heymach, MD, PhD, chair of the Thoracic/Head & Neck Medical Oncology at the MD Anderson Cancer Center, in a press release.2 “Our study gives hope for not only a potentially beneficial treatment option, but for a new level of precision to better target EGFR exon 20 mutations and to design more effective clinical trials.”
A new drug application for poziotinib was accepted by the FDA for patients with advanced or metastatic NSCLC harboring HER2 exon 20 insertion mutations in February 2022. Poziotinib also received fast track designation from the FDA in March 2021 for patients with HER2 exon 20 insertion–mutated NSCLC.
#Breast Cancer#Metastatic Breast Cancer#Increased Drug Resistance#Adenocarcinoma of Lung Stage IV#Adenocarcinoma of Lung Stage IIIB#polypeptide#weight loss#tirzepatide#semaglutide#bodybuilding#phcoker#Poziotinib#Poziotinib powder#Poziotinib cancer#Poziotinib supply
0 notes
Text
Tremelimumab
(Light chain)DIQMTQSPSS LSASVGDRVT ITCRASQSIN SYLDWYQQKP GKAPKLLIYA ASSLQSGVPSRFSGSGSGTD FTLTISSLQP EDFATYYCQQ YYSTPFTFGP GTKVEIKRTV AAPSVFIFPPSDEQLKSGTA SVVCLLNNFY PREAKVQWKV DNALQSGNSQ ESVTEQDSKD STYSLSSTLTLSKADYEKHK VYACEVTHQG LSSPVTKSFN RGEC(Heavy chain)QVQLVESGGG VVQPGRSLRL SCAASGFTFS SYGMHWVRQA PGKGLEWVAV IWYDGSNKYYADSVKGRFTI SRDNSKNTLY LQMNSLRAED TAVYYCARDP RGATLYYYYY GMDVWGQGTTVTVSSASTKG…
View On WordPress
#Anti-CTLA4 antibody#Antineoplastic#APPROVALS 2022#CP 675206#FDA 2022#Imjudo#Immune checkpoint inhibitor#peptide#Tremelimumab
0 notes
Text
The Antineoplastic Drugs Market in 2023 is US$ 134.66 billion, and is expected to reach US$ 338.70 billion by 2031 at a CAGR of 12.22%.
0 notes
Text
Oncology Coding Essentials: Understanding the Basics
Oncology, the branch of medicine focused on the diagnosis and treatment of cancer, presents unique challenges for medical coding and billing. Given the complexity of cancer diagnoses, a wide variety of treatment modalities, and evolving healthcare regulations, accurate and complete coding is essential to ensure appropriate reimbursement and compliance. This article aims to provide a comprehensive overview of the basics of coding in oncology, covering key concepts, common procedures, and best practices to help healthcare professionals navigate the complexities of coding in oncology.
Understanding Oncology Coding
Oncology coding involves the assignment of alphanumeric codes to describe the diagnosis, procedures, and services related to the treatment of patients with cancer. These codes are used for billing purposes, medical record documentation, and communication between healthcare providers, payers, and regulatory agencies. Proper coding ensures that healthcare services are accurately documented and reimbursed while meeting regulatory guidelines such as those established by the American Medical Association (AMA) and the Centers for Medicare and Medicaid Services (CMS).
Key Components of Oncology Coding
ICD-10-CM codes (International Classification of Diseases, Tenth Revision, Clinical Modification):
The ICD-10-CM coding system is used to classify and code diagnoses associated with cancer. Each diagnosis is assigned a unique code, usually consisting of alphanumeric characters, that provides detailed information about the type, location, and stage of the cancer. Proper selection of ICD-10-CM codes is essential to accurately document the patient's condition and determine appropriate treatment strategies.
CPT Codes (Current Procedural Terminology):
CPT codes are used to describe medical procedures and oncology billing services performed during cancer diagnosis and treatment. In oncology, CPT codes cover a wide range of services, including the administration of chemotherapy, radiation therapy, surgical procedures, diagnostic imaging, and laboratory tests. Healthcare providers must accurately report CPT codes to ensure proper reimbursement for services provided.
HCPCS Level II (Healthcare Common Procedure Coding System) Codes:
HCPCS Level II codes are used to report supplies, equipment, and other healthcare services not included in the CPT code set. In oncology, HCPCS Level II codes can be used to bill for durable medical equipment (DME), chemotherapy drugs, biologic agents, and other specialized treatments. Proper documentation and coding of HCPCS Level II codes are essential for accurate billing and reimbursement.
Common Oncology Procedures and Coding Guidelines
Chemotherapy Administration:
Chemotherapy administration involves the intravenous infusion or injection of antineoplastic drugs to treat cancer. CPT codes for chemotherapy administration are classified according to the route of administration (e.g., intravenous, intramuscular, subcutaneous) and whether the drug is given as a single agent or in combination with other drugs. Healthcare providers should document medications administered, route of administration, and duration of infusion to ensure accurate coding and billing.
Radiotherapy:
Radiation therapy is a common treatment modality for cancer that uses high-energy radiation to attack and destroy cancer cells. CPT codes for radiation therapy include codes for simulation, treatment administration, and management of treatment-related side effects. Proper documentation of the radiation treatment plan, delivery techniques, and treatment fractions is essential for accurate coding and reimbursement.
Surgical procedures:
Surgical procedures are often performed as part of cancer treatment to remove tumors, biopsy suspicious lesions, or reconstruct damaged tissue. CPT codes for surgical procedures in oncology include codes for excision, biopsy, reconstruction, and other related services. Healthcare providers should document the specific procedure performed, the anatomical site involved, and any associated findings or complications to ensure accurate coding and billing.
Diagnostic Imaging and Laboratory Tests:
Diagnostic imaging and laboratory tests play a crucial role in diagnosing, staging, and monitoring response to cancer treatment. CPT codes for imaging studies (e.g., CT scans, MRIs, PET scans) and laboratory tests (e.g., tumor markers, genetic testing) are used to report these services. Proper documentation of the type of imaging study or laboratory test performed, the anatomical site studied or analyzed, and the reason for the study is essential for accurate coding and reimbursement.
Best Practices for Oncology Coding
Stay up to Date with Coding Guidelines:
Healthcare providers and coding professionals should stay abreast of changes to coding guidelines, including updates to the ICD-10-CM, CPT, and HCPCS code sets. Regular training and education sessions can help ensure that coding practices align with current guidelines and regulatory requirements.
Document Complete and Accurate Patient Records:
Complete documentation is essential for accurate oncology coding and billing in oncology. Healthcare providers must document detailed information about the patient's cancer diagnosis, treatment plan, and response to treatment. Clear and complete documentation supports the assignment of appropriate codes and helps justify the medical necessity of the services provided.
Use EHR Systems and Coding Software:
Electronic health record (EHR) systems and coding software can streamline the coding process and help ensure consistency and accuracy in code selection. These tools typically include built-in coding reference guides, alerts for potential coding errors, and automated coding suggestions based on clinical documentation.
Audits and Periodic Reviews:
Conducting regular audits and reviews of coding practices can help identify coding errors, compliance issues, and areas for improvement. By reviewing coding accuracy, claim denials, and reimbursement rates, healthcare organizations can implement corrective actions to improve coding efficiency and revenue integrity.
Conclusion
Oncology coding is a complex and dynamic field that requires a deep understanding of coding principles, procedures, and guidelines. Accurate and complete coding is essential to ensure adequate reimbursement, compliance with regulatory requirements, and effective communication between healthcare providers, payers, and regulatory bodies.
By understanding the basics of oncology coding, healthcare professionals can navigate the complexities of cancer diagnosis and treatment while maximizing revenue and maintaining compliance with coding regulations. With a commitment to providing continuing education, documentation best practices, and utilization of coding technology, healthcare organizations can optimize their coding processes and provide high-quality care to cancer patients.
#Oncology Coding#Oncology Billing and Coding#Oncology Billing#Oncology Billing Services#Oncology Coding and Billing
0 notes
Text
Export Pharmaceutical Products from India
OLGud Pharmaceuticals export pharmaceutical products worldwide according to the requirements of international buyers. We export medicines in 20+ categories including antidiabetics, antineoplastic, anti-TB, cardiac, gastrointestinal, gynaec, pain, respiratory, and more. Get in touch with us for the fastest delivery at the best price.
#exportofpharmaceuticalproductsfromIndia#exportpharmaceuticalproducts#exportpharmaceuticalproductsfromIndia#pharmaproductexporterinIndia#pharmaceuticalexportersinIndia#PharmaExportCompanyInIndia#pharmaceuticalmedicinesexporterinIndia#OLGudPharmaceuticals
0 notes
Text
Tech Sheet - Cabozantinib (S)-malate API | API
0 notes
Text
As cancer immunotherapies revolutionize oncology treatment paradigms, proper coding is essential for ensuring accurate billing, reimbursement, and data analysis. We break down key ICD-10 codes for immunotherapy infusions, critical for both providers ...
#Mirari #MirariDoctor #MirariColdPlasma #ColdPlasma
0 notes
Text
Adagrasib
Adagrasib
FormulaC32H35ClFN7O2cas 2326521-71-3Mol weight604.1174
Antineoplastic DiseaseNon-small cell lung cancer
2022/12/12
FDA APPROVED, KRAZATI (Mirati Therapeutics)
MRTX-849
MRTX849
KRAS G12C inhibitor MRTX849
Adagrasib, sold under the brand name Krazati, is an anticancer medication used to treat non-small cell lung cancer.[1][2] Adagrasib is an inhibitor of the RAS GTPase…
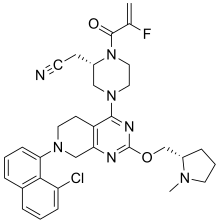
View On WordPress
2 notes
·
View notes
Text
Cytotoxic Safety Cabinet
A Cytotoxic Safety Cabinet is a specialized containment device designed to provide a controlled and secure environment for handling hazardous materials, particularly cytotoxic or antineoplastic agents. These cabinets are crucial in research laboratories, pharmaceutical facilities, and healthcare settings where the manipulation of cytotoxic drugs is necessary, as these drugs can pose significant risks to human health

0 notes