#Xenon tetrafluoride
Text
Figure 5.19 summarises the shapes of molecules with six sets of electron pairs.
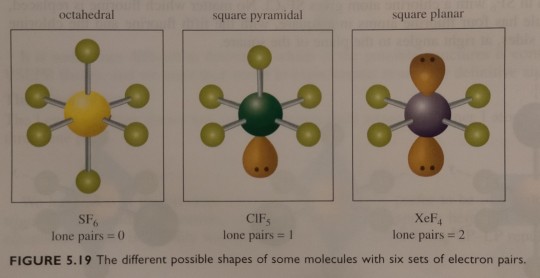
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#molecules#electron pairs#octahedral#square pyramidal#square planar#geometry#sulfur hexafluoride#chlorine pentafluoride#xenon tetrafluoride#sulfur#fluorine#chlorine#xenon
2 notes
·
View notes
Link
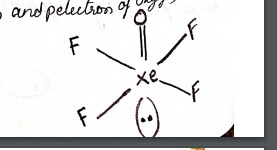
Here we discussed the XeF4 Lewis Structure. Xenon tetrafluoride is a chemical compound having the chemical formula XeF4. It is formed by the reaction of xenon with fluorine. Xenon tetrafluoride is a white-colored solid compound with a square planar shape. Xenon tetrafluoride is non polar and has zero dipole moment.
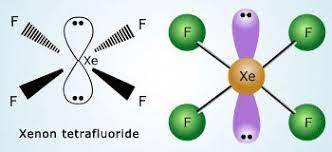
According to VSEPR theory in xenon tetrafluoride, the xenon center has two lone pairs. White crystalline colorless solid and sublimes at 115.7 ℃.
3 notes
·
View notes
Text

1 note
·
View note
Text
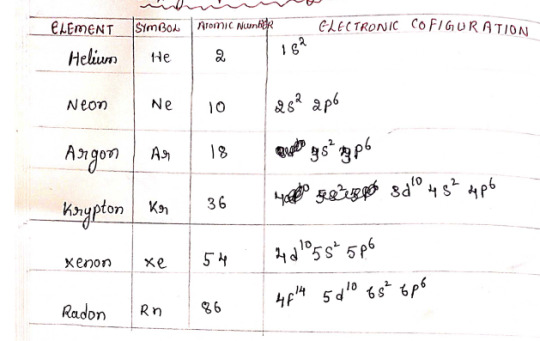
Zero Group Elements
Characteristics:
All non-metals
Have full outermost energy level making them very stable and unreactive.
Colorless, odorless gases at room temp
Outer most config. is ns²sp⁶ (except Helium - 1s²)
Called noble gases since they have no tendency to lose/gain electrons because of completely filled outer shell. Therefore they usually under normal conditions do not participate in chemical reactions, they are inert.
At STP, all are gases.
Oxides of Xenon
1) XeO3 - Xenon trioxide
Preparation:
i) Hydrolysis of XeF4 or XeF6: XeF6 + 3H2O -> XeO3 + 6HF
ii) From XeO2F2: XeO2F2 + H2O -> XeO3 + 2HF
Properties:
i) XeO3 + water -> weakly acidic sol: XeO3 + 3H2O -> H⁺ + H5XeO6⁻ (Xenic acid)
ii) Undergoes complete hydrolysis with alkali to produce perxenates: 2XeO3 + 4NaOH + 6H2O -> Xe + O2 +Na4XeO6.8H2O
iii) Strong oxidizing agent: XeO3 + 6KI + 6HCl -> Xe + 3I2 + 6KCl + 3H2O
Structure:
Hybridization: sp³
3 bond pairs + 1 lone pair = 4 CN
Geometry: pyramidal
O.S. of Xenon: +6

2) XeO4 - Xenon tetra oxide
Prep.
By the action of conc. H2SO4 on sodium or barium xenate at room temp: Na4XeO6 + 2H2SO4 -> XeO4 + 2Na2SO4 + 2H2O
Prop.
Is it very unstable, decomposes to form xenon and oxygen.
Structure
Hybridization: sp³
4 bond pairs = 4 CN
4 pi bonds formed by 4 unpaired electrons in d-orbital
Geometry: tetrahedral
O.S. of Xe: +8

Fluorides of Xenon
1) XeF2 - Xenon difluoride
prep
i) in a Ni vessel at 400*C at 1 atm: Xe + F2 -> XeF2
ii) With electric discharge: Xe + F2 -> XeF2
prop
Hydrolysis: 2XeF2 + 2H2O -> 2Xe + 4HF +O2
On heating: 2XeF2 -> XeF4 + Xe
On reduction: XeF2 +H2 -> Xe + 2HF
Structure
Hybridization: sp³d
2 bond pairs + 3 lone pairs = 5 CN
Geometry: linear
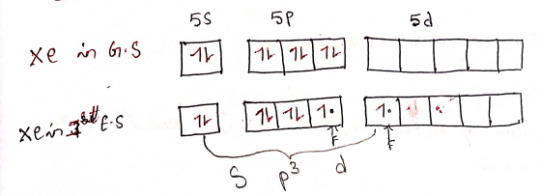
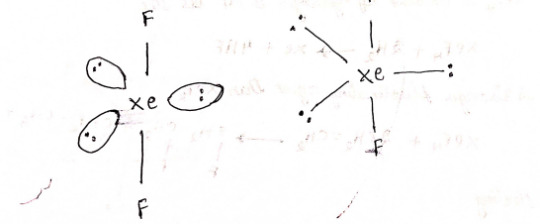
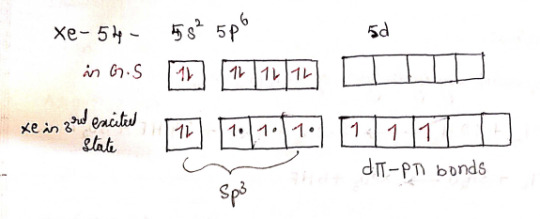
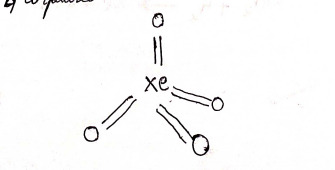
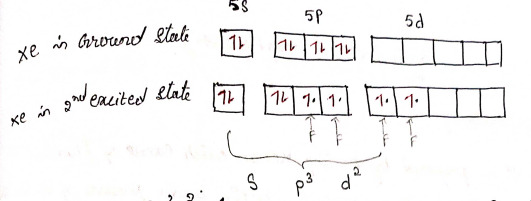
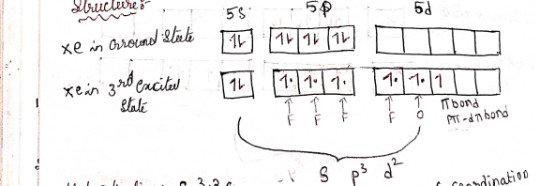
2) XeF4 - Xenon tetrafluoride
prep
Xenon and fluorine at 400*C and 5-6 atm: Xe +2F2 -> XeF4
prop
Oxidization: XeF4 + 2H2 -> Xe + 4HF
Heating: 3XeF4 -> 2XeF6
Hydration: 6XeF4 +12H2O -> 2XeO3 + 4Xe + 2HF + 3O2
structure
Hybridization: sp³d²
4 bond pairs + 2 lone pairs = 6 CN
Geometry: Square planar

3) XeF6 - Xenon hexafluoride
prep
i) xenon+fluorine at 250-300*C under 50-60atm: Xe +3F2 -> XeF6
ii) Oxidation of XeF4 with O2F2: XeF4 + O2F2 -> XeF6 + O2
prop
With HF: XeF6 + HF -> XeHF6
With H2: XeF6 + 3H2 -> Xe + 6HF
With H2O: XeF6 + H2O -> XeOF4 + 2HF, XeF6 + 3H2O -> XeO3 + 2HF
structure
Hybridization: sp³d³
6 bond pairs + 1 lone pair = 7 CN
Geometry: Distorted octahedron


Oxyfluorides
XeOF4 - Xenon Oxytetrafluroide
Preparation
i) On hydrolysis of XeF6: XeF6 + H2O -> XeOF4 + 2HF
ii) By the action of XeF6 on SiO2: 2XeF6 + SiO2 -> XeOF4 + SiF4
Properties
Colorless solid, melting point of -46*C
Reduced by H to Xe: XeOF4 + 3H2 -> Xe + H2O +4HF
Structure
Hybridization: sp³d²
5 bond pairs + 1 lone pair = 6 CN
Geometry: Square pyramidal
Has one pi bond with oxygen atom
0 notes
Text
so like we got an international student boarding in our spare room for the year and he’s trying to choose a new english name and i really wanna convince my parents n him to choose xenon as a name
#probably not gonna happen but like#it would just be cool you know#apparently he was given his english name when he was like six so he wants to change it. his friends have apparently also nicknamed him jinx#which is. also admittedly kind of cool. but not like. given name material. that's def nickname material but not given name#honestly?? i would name MY kid xenon that would be so cool. cooler than carbon tetrafluoride *coughs @my first year chem teacher*
0 notes
Photo

Xenon tetrafluoride is a Chemical compound with chemical formula XeF4. It is produced by the chemical reaction of xenon of fluorine. They make a formation in the single square plane. So, it is proved that the XeF4 is nonpolar. More details read an article.
0 notes
Text
Fluorine Derivatives Market 2021 SWOT Analysis by Forecast to 2027
In the latest report, with a graph of the Fluorine Derivatives market, the assessment bases fundamentally available examples, demand reach, and future odds of this territory over the gauge time period. Besides, the report gives a nitty gritty measurable outline as far as patterns portraying the geographic chances and ventures of driving business investors.
A definite outline of the worldwide market size, territorial and nation market size, market development division, piece of the overall industry, serious climate, stock levels, homegrown and worldwide market player sway, store network streamlining, import requirements, most recent patterns, opportunity examination, and vital market development is introduced in the market report on Fluorine Derivatives.
Free Sample Report + All Related Graphs & Charts (Including COVID19 Impact Analysis) @:https://www.crystalmarketresearch.com/report-sample/CM0114678
Some of the key players in the Global Fluorine Derivatives market are:
Solvay
Pelchem
Honeywell
Navin Fluorine International
Kanto Denka
Air Products and Chemicals
Advance Research Chemicals
Linde
Fluorine Derivatives Market
Continue...
TOC of Fluorine Derivatives Market Report:
Industry Overview of Fluorine Derivatives Market.
Accumulation Cost Structure Analysis of Fluorine Derivatives Market.
Specific Information and Manufacturing Plants Analysis of Fluorine Derivatives Market.
Limit, Production, and Revenue Analysis.
Worth, Cost, Gross and Gross Margin Analysis of Fluorine Derivatives Market by Regions, Types, and Manufacturers.
Use Volume, Consumption Value, and Sale Price Analysis of Fluorine Derivatives Market industry by Regions, Types, and Applications.
Supply, Import, Export, and Consumption Analysis of Fluorine Derivatives Market.
Huge Manufacturers Analysis of Fluorine Derivatives Market industry.
Publicizing Trader or Distributor Analysis of Fluorine Derivatives Market.
Industry Chain Analysis of Fluorine Derivatives Market.
Progression Trend Analysis of Fluorine Derivatives Market.
New Project Investment Feasibility Analysis of Fluorine Derivatives Market.
Request Discount on this Report @https://www.crystalmarketresearch.com/check-discount/CM0114678
Prominent Points in Fluorine Derivatives Market Businesses Segmentation:
Fluorine Derivatives Market , By Type, Estimates and Forecast, 2016-2027 ($Million)
Monofluoride
Hydrogen Fluoride
Xenon Hexafluoroplatinate
Xenon Difluoride
Tetrafluoride
Hexafluoride
Others
Fluorine Derivatives Market , By Application, Estimates and Forecast, 2016-2027 ($Million)
Nuclear Fuels
Glass And Ceramics
Propellants Pharmaceuticals
Fire Extinguishers
Others
Fluorine Derivatives Market
Regions & Countries Mentioned In The Global Fluorine Derivatives Market Report::
North America Region
Europe Region
Asia-Pacific Region
South America Region
The Middle East & Africa Region
Motivations to buy the exploration report:
Gives inside and out research examination of the general Fluorine Derivatives market. which can help save time for business people hoping to begin business in regards to the Fluorine Derivatives Market.
Different moving news, gauge examination and key contenders of the market are effectively accessible with all the vital data.
Whole market extension and data can be accessible at the fingertips for any business person or organization that buys the report which can help a new business or a contender comprehend the Fluorine Derivatives Market in detail with every one of the important components.
Diagrams, pie outlines and different portrayals that can assist the peruser with understanding the data at a solitary look.
All vital data with respect to the market that can assist a maker with understanding the purchaser conduct, business sections and sell items dependent on the examination data.
Most moving Coronavirus pandemic effect available and industry with all the important recuperation examination.
Fluorine Derivatives Research Report Inspects:
Item Type and Applications
Coronavirus Impact investigation
Vital participants/organizations of Fluorine Derivatives Market all around the world
You can Buy This Report from Here @https://www.crystalmarketresearch.com/send-an-enquiry/CM0114678
Contacts Us:
Crystal Market Research
Sherry | APAC Marketing Division: Level 23-1
Premier Suite, Mont Kiara, 50480 Kuala
Lumpur, Malaysia
E-mail: [email protected]
0 notes
Text
Fluorine Derivatives Market Value Chain Analysis and Forecast up to 2025
Amongst all of the chemical elements, fluorine is the most reactive & electronegative. Fluorine reacts with organic and inorganic substances. Fluorine derivatives are obtained by compounds with the help of chemical reaction. Compounds are synthesized with the help of molecules with higher absorption wavelengths in order to obtain compounds with the finest properties of fluorinated derivatives. Direct fluorination of compounds in acidic conditions results in production of its mono-, di- and trifluorinated derivatives at mild yields. Reactions between elementary fluorine and complex organic molecules usually result in multiple nonspecific processes due to the high enthalpy of C-H bond fluorination, which exceeds the energy of carbon-based single bonds and causes destruction of the target molecule.
The fluorine derivatives market is driven by large scale industries as it have high demand for the fluorine because it is lightweight and cost effective over gas. Increase in number of end-users of fluorine derivatives such as uranium hexafluoride is also anticipated to boost the global consumption of fluorine derivatives. Rise in end-use of sulfur hexafluoride is also driving the global fluorine derivatives market. Demand for fluorine derivatives is estimated to decline in the near future because while transportation if the gas is leaked it causes harmful hazardous effects. Furthermore, complex production process discourages the arrival of new entrants. This also poses a challenge for existing manufacturers of fluorine derivatives.
Based on application, the fluorine derivatives market can be segmented into nuclear fuels, glass & ceramics manufacturing, refrigerants, propellants pharmaceuticals, and fire extinguishers. The nuclear fuels segment accounted for the major share of the global fluorine derivatives market in terms of revenue in 2016. The replacement of monovalent fluorine for divalent bridging oxygen led to the formation of Si (O3F), which leads to decrease in melt viscosity and weakening of the glass structure. Consequently, fluorine additions are used as binding agent in the melting of many commercial glasses.
Request For the Customization @ https://www.transparencymarketresearch.com/sample/sample.php?flag=CR&rep_id=38822
The global fluorine derivatives market can be segregated based on compounds formed. Fluoride forms derivatives in combination with metals, nonmetals, metalloids, and most noble gases. Alkali metals form ionic and highly soluble monofluoride derivatives. Hydrogen and fluorine combine to produce hydrogen fluoride derivatives. Noble gases combine to form xenon hexafluoroplatinate, xenon difluoride, tetrafluoride, hexafluoride, and multiple oxyfluorides. Among other noble gases, krypton forms difluoride derivatives. The carbon–fluorine bond is organic chemistry’s strongest bond, and gives stability to organofluorines derivatives.
In terms of geography, the fluorine derivatives market can be divided into North America, Asia Pacific, Europe, Middle East & Africa, and Latin America. In terms of revenue, the fluorine derivatives market in North America is estimated to exhibit steady growth during the forecast period. The market in Europe is projected to expand at a sluggish pace in terms of revenue. Asia Pacific is likely to lead the fluorine derivatives market in terms of production and demand. Growth in urbanization and favorable manufacturing regulations are boost the market in Asia Pacific. On the other hand, the demand for fluorine derivatives is expected to be low in Middle East & Africa and Latin America during the forecast period.
Get PDF Brochure for more Professional & Technical industry insights: https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=38822
Key players operating in the fluorine derivatives market are Solvay S.A., Pelchem SOC Ltd., Navin Fluorine International Limited, KANTO DENKA KOGYO CO., LTD., Air Products & Chemicals, Inc., Advance Research Chemicals, Inc., and Linde AG.
0 notes
Text
Fluorine Derivatives Market to Witness Exponential Growth by 2025
Amongst all of the chemical elements, fluorine is the most reactive & electronegative. Fluorine reacts with organic and inorganic substances. Fluorine derivatives are obtained by compounds with the help of chemical reaction. Compounds are synthesized with the help of molecules with higher absorption wavelengths in order to obtain compounds with the finest properties of fluorinated derivatives. Direct fluorination of compounds in acidic conditions results in production of its mono-, di- and trifluorinated derivatives at mild yields. Reactions between elementary fluorine and complex organic molecules usually result in multiple nonspecific processes due to the high enthalpy of C-H bond fluorination, which exceeds the energy of carbon-based single bonds and causes destruction of the target molecule.
Read Report Overview @
https://www.transparencymarketresearch.com/fluorine-derivatives-market.html
The fluorine derivatives market is driven by large scale industries as it have high demand for the fluorine because it is lightweight and cost effective over gas. Increase in number of end-users of fluorine derivatives such as uranium hexafluoride is also anticipated to boost the global consumption of fluorine derivatives. Rise in end-use of sulfur hexafluoride is also driving the global fluorine derivatives market. Demand for fluorine derivatives is estimated to decline in the near future because while transportation if the gas is leaked it causes harmful hazardous effects. Furthermore, complex production process discourages the arrival of new entrants. This also poses a challenge for existing manufacturers of fluorine derivatives.
Based on application, the fluorine derivatives market can be segmented into nuclear fuels, glass & ceramics manufacturing, refrigerants, propellants pharmaceuticals, and fire extinguishers. The nuclear fuels segment accounted for the major share of the global fluorine derivatives market in terms of revenue in 2016. The replacement of monovalent fluorine for divalent bridging oxygen led to the formation of Si (O3F), which leads to decrease in melt viscosity and weakening of the glass structure. Consequently, fluorine additions are used as binding agent in the melting of many commercial glasses.
The global fluorine derivatives market can be segregated based on compounds formed. Fluoride forms derivatives in combination with metals, nonmetals, metalloids, and most noble gases. Alkali metals form ionic and highly soluble monofluoride derivatives. Hydrogen and fluorine combine to produce hydrogen fluoride derivatives. Noble gases combine to form xenon hexafluoroplatinate, xenon difluoride, tetrafluoride, hexafluoride, and multiple oxyfluorides. Among other noble gases, krypton forms difluoride derivatives. The carbon–fluorine bond is organic chemistry's strongest bond, and gives stability to organofluorines derivatives.
In terms of geography, the fluorine derivatives market can be divided into North America, Asia Pacific, Europe, Middle East & Africa, and Latin America. In terms of revenue, the fluorine derivatives market in North America is estimated to exhibit steady growth during the forecast period. The market in Europe is projected to expand at a sluggish pace in terms of revenue. Asia Pacific is likely to lead the fluorine derivatives market in terms of production and demand. Growth in urbanization and favorable manufacturing regulations are boost the market in Asia Pacific. On the other hand, the demand for fluorine derivatives is expected to be low in Middle East & Africa and Latin America during the forecast period.
Request to view Sample Report:
https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=38822
Key players operating in the fluorine derivatives market are Solvay S.A., Pelchem SOC Ltd., Navin Fluorine International Limited, KANTO DENKA KOGYO CO., LTD., Air Products & Chemicals, Inc., Advance Research Chemicals, Inc., and Linde AG.
0 notes
Text
Fluorine Derivatives Market to Observe Strong Development by 2025
Amongst all of the chemical elements, fluorine is the most reactive & electronegative. Fluorine reacts with organic and inorganic substances. Fluorine derivatives are obtained by compounds with the help of chemical reaction. Compounds are synthesized with the help of molecules with higher absorption wavelengths in order to obtain compounds with the finest properties of fluorinated derivatives. Direct fluorination of compounds in acidic conditions results in production of its mono-, di- and trifluorinated derivatives at mild yields. Reactions between elementary fluorine and complex organic molecules usually result in multiple nonspecific processes due to the high enthalpy of C-H bond fluorination, which exceeds the energy of carbon-based single bonds and causes destruction of the target molecule.
View Report Preview:
https://www.transparencymarketresearch.com/fluorine-derivatives-market.html
The fluorine derivatives market is driven by large scale industries as it have high demand for the fluorine because it is lightweight and cost effective over gas. Increase in number of end-users of fluorine derivatives such as uranium hexafluoride is also anticipated to boost the global consumption of fluorine derivatives. Rise in end-use of sulfur hexafluoride is also driving the global fluorine derivatives market. Demand for fluorine derivatives is estimated to decline in the near future because while transportation if the gas is leaked it causes harmful hazardous effects. Furthermore, complex production process discourages the arrival of new entrants. This also poses a challenge for existing manufacturers of fluorine derivatives.
Based on application, the fluorine derivatives market can be segmented into nuclear fuels, glass & ceramics manufacturing, refrigerants, propellants pharmaceuticals, and fire extinguishers. The nuclear fuels segment accounted for the major share of the global fluorine derivatives market in terms of revenue in 2016. The replacement of monovalent fluorine for divalent bridging oxygen led to the formation of Si (O3F), which leads to decrease in melt viscosity and weakening of the glass structure. Consequently, fluorine additions are used as binding agent in the melting of many commercial glasses.
The global fluorine derivatives market can be segregated based on compounds formed. Fluoride forms derivatives in combination with metals, nonmetals, metalloids, and most noble gases. Alkali metals form ionic and highly soluble monofluoride derivatives. Hydrogen and fluorine combine to produce hydrogen fluoride derivatives. Noble gases combine to form xenon hexafluoroplatinate, xenon difluoride, tetrafluoride, hexafluoride, and multiple oxyfluorides. Among other noble gases, krypton forms difluoride derivatives. The carbon–fluorine bond is organic chemistry’s strongest bond, and gives stability to organofluorines derivatives.
In terms of geography, the fluorine derivatives market can be divided into North America, Asia Pacific, Europe, Middle East & Africa, and Latin America. In terms of revenue, the fluorine derivatives market in North America is estimated to exhibit steady growth during the forecast period. The market in Europe is projected to expand at a sluggish pace in terms of revenue. Asia Pacific is likely to lead the fluorine derivatives market in terms of production and demand. Growth in urbanization and favorable manufacturing regulations are boost the market in Asia Pacific. On the other hand, the demand for fluorine derivatives is expected to be low in Middle East & Africa and Latin America during the forecast period.
Key players operating in the fluorine derivatives market are Solvay S.A., Pelchem SOC Ltd., Navin Fluorine International Limited, KANTO DENKA KOGYO CO., LTD., Air Products & Chemicals, Inc., Advance Research Chemicals, Inc., and Linde AG.
Request to view Sample Report:
https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=38822
The report offers a comprehensive evaluation of the market. It does so via in-depth qualitative insights, historical data, and verifiable projections about market size. The projections featured in the report have been derived using proven research methodologies and assumptions. By doing so, the research report serves as a repository of analysis and information for every facet of the market, including but not limited to: Regional markets, technology, types, and applications.
0 notes
Text
Table 5.4 summarises the relationships between number of sets of electron pairs, the geometry of the sets of electron pairs and molecular shape.
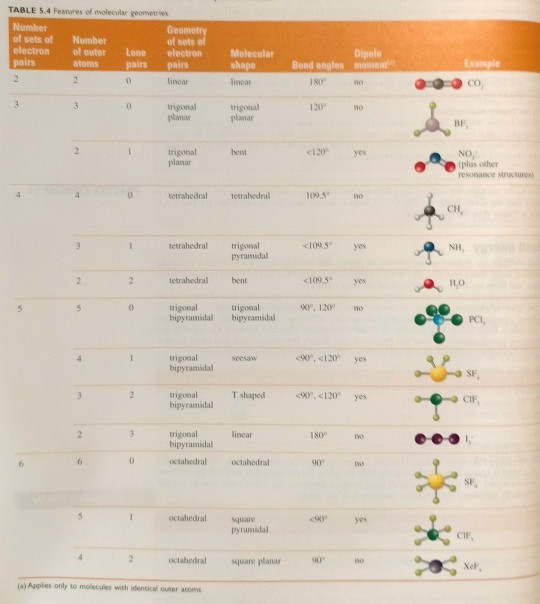
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#summary#relationship#electrons#geometry#linear#trigonal planar#bent#tetrahedral#trigonal bipyramidal#seesaw#t shape#octahedral#square pyramidal#square planar#xenon tetrafluoride#chlorine pentafluoride#sulfur hexafluoride#triiodide#chlorine trifluoride#sulfur tetrafluoride#phosphorus pentachloride#water#ammonia#methane#nitrogen dioxide#boron trifluoride
0 notes
Text
Fluorine Derivatives Market to Record an Exponential CAGR by 2025
Amongst all of the chemical elements, fluorine is the most reactive & electronegative. Fluorine reacts with organic and inorganic substances. Fluorine derivatives are obtained by compounds with the help of chemical reaction. Compounds are synthesized with the help of molecules with higher absorption wavelengths in order to obtain compounds with the finest properties of fluorinated derivatives. Direct fluorination of compounds in acidic conditions results in production of its mono-, di- and trifluorinated derivatives at mild yields. Reactions between elementary fluorine and complex organic molecules usually result in multiple nonspecific processes due to the high enthalpy of C-H bond fluorination, which exceeds the energy of carbon-based single bonds and causes destruction of the target molecule.
Read Report Overview @
https://www.transparencymarketresearch.com/fluorine-derivatives-market.html
The fluorine derivatives market is driven by large scale industries as it have high demand for the fluorine because it is lightweight and cost effective over gas. Increase in number of end-users of fluorine derivatives such as uranium hexafluoride is also anticipated to boost the global consumption of fluorine derivatives. Rise in end-use of sulfur hexafluoride is also driving the global fluorine derivatives market. Demand for fluorine derivatives is estimated to decline in the near future because while transportation if the gas is leaked it causes harmful hazardous effects. Furthermore, complex production process discourages the arrival of new entrants. This also poses a challenge for existing manufacturers of fluorine derivatives.
Based on application, the fluorine derivatives market can be segmented into nuclear fuels, glass & ceramics manufacturing, refrigerants, propellants pharmaceuticals, and fire extinguishers. The nuclear fuels segment accounted for the major share of the global fluorine derivatives market in terms of revenue in 2016. The replacement of monovalent fluorine for divalent bridging oxygen led to the formation of Si (O3F), which leads to decrease in melt viscosity and weakening of the glass structure. Consequently, fluorine additions are used as binding agent in the melting of many commercial glasses.
The global fluorine derivatives market can be segregated based on compounds formed. Fluoride forms derivatives in combination with metals, nonmetals, metalloids, and most noble gases. Alkali metals form ionic and highly soluble monofluoride derivatives. Hydrogen and fluorine combine to produce hydrogen fluoride derivatives. Noble gases combine to form xenon hexafluoroplatinate, xenon difluoride, tetrafluoride, hexafluoride, and multiple oxyfluorides. Among other noble gases, krypton forms difluoride derivatives. The carbon–fluorine bond is organic chemistry's strongest bond, and gives stability to organofluorines derivatives.
In terms of geography, the fluorine derivatives market can be divided into North America, Asia Pacific, Europe, Middle East & Africa, and Latin America. In terms of revenue, the fluorine derivatives market in North America is estimated to exhibit steady growth during the forecast period. The market in Europe is projected to expand at a sluggish pace in terms of revenue. Asia Pacific is likely to lead the fluorine derivatives market in terms of production and demand. Growth in urbanization and favorable manufacturing regulations are boost the market in Asia Pacific. On the other hand, the demand for fluorine derivatives is expected to be low in Middle East & Africa and Latin America during the forecast period.
Request to view Sample Report:
https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=38822
Key players operating in the fluorine derivatives market are Solvay S.A., Pelchem SOC Ltd., Navin Fluorine International Limited, KANTO DENKA KOGYO CO., LTD., Air Products & Chemicals, Inc., Advance Research Chemicals, Inc., and Linde AG.
0 notes