Link
Here we discussed the XeF4 Lewis Structure. Xenon tetrafluoride is a chemical compound having the chemical formula XeF4. It is formed by the reaction of xenon with fluorine. Xenon tetrafluoride is a white-colored solid compound with a square planar shape. Xenon tetrafluoride is non polar and has zero dipole moment.
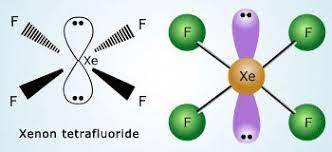
According to VSEPR theory in xenon tetrafluoride, the xenon center has two lone pairs. White crystalline colorless solid and sublimes at 115.7 ℃.
3 notes
·
View notes