#Pharmaceutical Contract Packaging Market news
Link
2 notes
·
View notes
Text
Outsourcing for Efficiency: Exploring Contract Manufacturing in Pharmaceuticals

The world of pharmaceuticals is a complex dance between research, development, and production. While bringing a new drug to market is a triumph of science, the manufacturing side can be a logistical beast. This is where contract manufacturing steps in, offering a valuable partnership for pharmaceutical companies.
What is Contract Manufacturing?
Imagine a company with a brilliant new drug formula. They’ve poured resources into research and development, but lack the facilities or expertise for large-scale production. This is where a Contract Manufacturing Organization (CMO) comes in. A CMO is a specialized company that manufactures drugs on behalf of other businesses. They handle everything from formulation to packaging, using their expertise and facilities to bring the drug to life.
The Advantages of Outsourcing Production
There are several reasons a pharmaceutical company might choose contract manufacturing:
Cost-Effectiveness: Building and maintaining a production facility is expensive. CMOs offer a cost-effective alternative, with economies of scale and existing infrastructure.
Focus on R&D: By outsourcing production, pharmaceutical companies can free up resources to focus on what they do best: research and development of new drugs.
Expertise and Flexibility: CMOs specialize in pharmaceutical manufacturing and have the expertise to handle complex projects. They can also offer flexibility, scaling production up or down as needed.
Speed to Market: Partnering with a CMO can significantly speed up the time it takes to get a drug to market.
The Different Players: CMOs vs. CDMOs
While CMOs handle the manufacturing process, some companies offer even more comprehensive services. A Contract Development and Manufacturing Organization (CDMO) takes things a step further. They can assist with everything from pre-formulation and development to clinical trials and, of course, final production.
Ensuring Quality and Collaboration
Contract manufacturing hinges on a strong partnership. Pharmaceutical companies need to ensure CMOs meet strict quality standards and regulations. Clear communication and robust quality control procedures are essential.
The Future of Contract Manufacturing
The pharmaceutical industry is constantly evolving. As the demand for new drugs and therapies grows, contract manufacturing is likely to play an even more crucial role. CMOs will continue to develop their expertise and capabilities, offering a wider range of services and keeping pace with technological advancements.
Conclusion
Contract manufacturing is a win-win for the pharmaceutical industry. It allows drug companies to focus on innovation while leveraging the expertise of CMOs to bring life-saving treatments to patients faster and more efficiently. As the industry moves forward, contract manufacturing will undoubtedly remain a key driver of progress.
0 notes
Text
Global Top 25 Companies Accounted for 61% of total Pharmaceutical Sterile Fill-Finish market (QYResearch, 2021)
In pharmaceutical manufacturing, fill-finish operations are critical, since fill-finish is the last step before a product is packaged and ultimately delivered to the patient. By the time a drug reaches this stage, the drug product is highly valuable, as it has already been through labour- and cost-intensive production stages, including upstream processing, cell culture or fermentation and downstream purification. Failures in the integrity of the fill-finish stage can introduce microbial contamination and generate issues with formulation and dosing.
According to the new market research report “Global Pharmaceutical Sterile Fill-Finish Market Report 2023-2029”, published by QYResearch, the global Pharmaceutical Sterile Fill-Finish market size is projected to reach USD 5.15 billion by 2029, at a CAGR of 10.2% during the forecast period.
Figure. Global Pharmaceutical Sterile Fill-Finish Market Size (US$ Million), 2018-2029
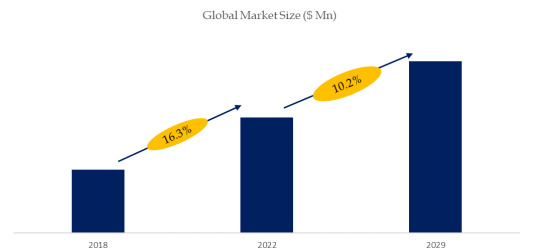
Above data is based on report from QYResearch: Global Pharmaceutical Sterile Fill-Finish Market Report 2023-2029 (published in 2023). If you need the latest data, plaese contact QYResearch..
Figure. Global Pharmaceutical Sterile Fill-Finish Top 25 Players Ranking and Market Share (Ranking is based on the revenue of 2022, continually updated)

Above data is based on report from QYResearch: Global Pharmaceutical Sterile Fill-Finish Market Report 2023-2029 (published in 2023). If you need the latest data, plaese contact QYResearch.
The global key manufacturers of Pharmaceutical Sterile Fill-Finish include Baxter BioPharma Solutions, Boehringer Ingelheim, Fresenius Kabi, Aenova, Pfizer CentreOne, Vetter Pharma, WuXi Biologics, Jubilant HollisterStier, LSNE Contract Manufacturing, Bushu Pharmaceuticals, etc. In 2022, the global top 10 players had a share approximately 61.0% in terms of revenue.
About QYResearch
QYResearch founded in California, USA in 2007.It is a leading global market research and consulting company. With over 16 years’ experience and professional research team in various cities over the world QY Research focuses on management consulting, database and seminar services, IPO consulting, industry chain research and customized research to help our clients in providing non-linear revenue model and make them successful. We are globally recognized for our expansive portfolio of services, good corporate citizenship, and our strong commitment to sustainability. Up to now, we have cooperated with more than 60,000 clients across five continents. Let’s work closely with you and build a bold and better future.
QYResearch is a world-renowned large-scale consulting company. The industry covers various high-tech industry chain market segments, spanning the semiconductor industry chain (semiconductor equipment and parts, semiconductor materials, ICs, Foundry, packaging and testing, discrete devices, sensors, optoelectronic devices), photovoltaic industry chain (equipment, cells, modules, auxiliary material brackets, inverters, power station terminals), new energy automobile industry chain (batteries and materials, auto parts, batteries, motors, electronic control, automotive semiconductors, etc.), communication industry chain (communication system equipment, terminal equipment, electronic components, RF front-end, optical modules, 4G/5G/6G, broadband, IoT, digital economy, AI), advanced materials industry Chain (metal materials, polymer materials, ceramic materials, nano materials, etc.), machinery manufacturing industry chain (CNC machine tools, construction machinery, electrical machinery, 3C automation, industrial robots, lasers, industrial control, drones), food, beverages and pharmaceuticals, medical equipment, agriculture, etc.
0 notes
Text
Sustainable Pharmaceutical Packaging Market Size Global Trends, and Opportunities Forecast by 2031
Sustainable Pharmaceutical Packaging Market SizeScope & Overview
A competitive quadrant is included in the study, which is a patented method for analyzing and evaluating a company's position based on its industry position score and market performance score. The tool divides the players into four groups based on a variety of characteristics. Financial performance during the previous years, growth plans, innovation score, new product releases, investments, market share growth, and so on are some of the elements that are examined. The study provides a thorough analysis of the worldwide Sustainable Pharmaceutical PackagingMarket Size. In-depth qualitative research, verifiable data from reliable sources, and market size predictions are all included in the report. The estimates are based on well-established research methodology.
The Sustainable Pharmaceutical Packaging market report generated using a combination of primary and secondary sources. Interviews, questionnaires, and observation of recognized industry personnel are used in the primary research. The Ansoff Matrix and Porter's 5 Forces model are used to conduct an in-depth market study in the research. In addition, the research discusses the influence of Covid-19 on the market. The report also contains information on the industry's regulatory environment, which will assist you in making an informed decision. The paper goes over the major regulatory agencies as well as the major rules and regulations that have been established on this industry in different parts of the world. The study also includes a competition analysis utilizing the analyst's competitive positioning technique, Positioning Quadrants.
Get a Sample Report https://www.snsinsider.com/sample-request/2811
Market Key Players:
Vetter Pharma International, Schott AG, Amcor plc, Gerresheimer AG, AptarGroup Inc, Owens Illinois Inc, CCL Industries Inc, SGD Pharma, West Pharmaceutical Services Inc, Drug Plastics Group, WestRock Company, Becton, Dickinson, and Company
Market Segmentation
Market segmentation by product type, application, end-user, and geography is discussed in the Sustainable Pharmaceutical Packaging research report. The research looks into the industry's growth goals, cost-cutting measures, and production procedures. A full evaluation of the core industry, including categorization and definition, as well as the structure of the supply and demand chain, is also included in the study report.
By Raw Material:
Paper & Paperboards
Plastic & Polymers
Aluminium Foil
Glass
Others
By Product type:
Primary
Secondary
Tertiary
By End users:
Contract Packaging
Institutional Pharmacy
Retail Pharmacy
Pharma Manufacturing
Competitive Outlook
The study includes a thorough examination of the market's key players, including company profiles, SWOT analyses, recent developments, and business plans. The analysis looks at all aspects of the industry, with an emphasis on major players such market leaders, followers, and newcomers. Because it clearly illustrates competitive analysis of key competitors in the Sustainable Pharmaceutical Packaging market by product, price, financial status, product portfolio, growth strategies, and geographical presence, the research is an investor's guide.
Key Objectives of Sustainable Pharmaceutical Packaging Market Report
To examine the market in terms of growth trends, prospects, and their involvement in the whole industry.
Examine competition developments such as market expansions, agreements, new product launches, and acquisitions.
Examine and research the company's market size (volume and value), key regions/countries, products, and applications, as well as background information and forecasting.
Primary global market manufacturing firms, to define, clarify, and evaluate product sales volume, value, and market share, market rivalry landscape, SWOT analysis, and future development plans.
Buy the Research Report Now https://www.snsinsider.com/checkout/2811
About Us:
SNS Insider is one of the leading Market Size research and consulting agencies that dominates the Market Size research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate Market Size data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
0 notes
Text
Biopharmaceutical CMO Market Growth Opportunities and Competitive Landscape Report to 2033
Market Definition
A Contract Manufacturing Organization (CMO), also known as a Biopharmaceutical CMO, is a company that provides manufacturing and other services to the pharmaceutical and biotechnology industries. CMOs are an important part of the pharmaceutical supply chain, and they play a vital role in bringing new drugs and therapies to market.
CMOs specialize in the manufacture of active pharmaceutical ingredients (APIs) and finished dosage forms (FDFs). They also provide a range of other services, such as analytical testing, formulation development, and packaging. CMOs are typically large, multinational companies with extensive experience in drug manufacturing.
Market Outlook
The key trends in Biopharmaceutical CMO technology are:
1. The use of biotechnology to develop new drugs and therapies.
2. The use of cell culture and fermentation technologies to produce biopharmaceuticals.
3. The use of monoclonal antibodies and other protein-based drugs.
4. The use of nucleic acid-based drugs and gene therapy.
The biopharmaceutical CMO market is driven by the increasing demand for biopharmaceuticals, the need for specialized manufacturing facilities, and the increasing number of biopharmaceutical companies. The biopharmaceutical industry is growing at a rapid pace, and the number of biopharmaceutical companies is increasing. This is resulting in an increased demand for CMOs. CMOs are specialized manufacturing facilities that are required for the production of biopharmaceuticals. They are required to meet the stringent quality standards set by the FDA. The increasing number of biopharmaceutical companies is resulting in an increased demand for CMOs.
The biopharmaceutical CMO market is facing a number of key restraints and challenges. Firstly, the market is highly competitive and there are a large number of players operating in the space. This makes it difficult for new entrants to gain a foothold in the market. Secondly, the market is capital intensive and requires significant investment in research and development. This is a major barrier for small and medium sized companies. Thirdly, the regulatory environment is constantly changing and this makes it difficult for companies to keep up with the latest regulations. Finally, the market is reliant on a small number of key customers and this makes it difficult to diversify revenue streams.
To Know More: https://www.globalinsightservices.com/reports/biopharmaceutical-cmo-market/
Research Objectives
Estimates and forecast the overall market size for the total market, across product, service type, type, end-user, and region
Detailed information and key takeaways on qualitative and quantitative trends, dynamics, business framework, competitive landscape, and company profiling
Identify factors influencing market growth and challenges, opportunities, drivers and restraints
Identify factors that could limit company participation in identified international markets to help properly calibrate market share expectations and growth rates
Trace and evaluate key development strategies like acquisitions, product launches, mergers, collaborations, business expansions, agreements, partnerships, and R&D activities
Thoroughly analyze smaller market segments strategically, focusing on their potential, individual patterns of growth, and impact on the overall market
To thoroughly outline the competitive landscape within the market, including an assessment of business and corporate strategies, aimed at monitoring and dissecting competitive advancements.
Identify the primary market participants, based on their business objectives, regional footprint, product offerings, and strategic initiatives
Request Sample: https://www.globalinsightservices.com/request-sample/GIS21593/
Market Segmentation
The biopharmaceutical CMO market report is bifurcated on the basis of product, source, service, and region. On the basis of product, it is segmented into biologics and biosimilars. Based on source, it is analyzed across mammalian and non-mammalian. By service, it is categorized into contract manufacturing, process development, packaging, and others. Region-wise, it is studied across North America, Europe, Asia-Pacific, and rest of the World.
Request Customization@ https://www.globalinsightservices.com/request-customization/GIS21593/
Major Players
The biopharmaceutical CMO market report includes players such as Toyobo Co., Ltd., Samsung Biologics, Patheon, Lonza AG, WuXi Biologics, AbbVie Inc., Binex Co., Ltd., JRS Pharma, Biomeva GmbH, and ProBioGen AG.
Request Discounted Pricing@ https://www.globalinsightservices.com/request-special-pricing/GIS21593/
Research Scope
Scope – Highlights, Trends, Insights. Attractiveness, Forecast
Market Sizing – Product Type, End User, Offering Type, Technology, Region, Country, Others
Market Dynamics – Market Segmentation, Demand and Supply, Bargaining Power of Buyers and Sellers, Drivers, Restraints, Opportunities, Threat Analysis, Impact Analysis, Porters 5 Forces, Ansoff Analysis, Supply Chain
Business Framework – Case Studies, Regulatory Landscape, Pricing, Policies and Regulations, New Product Launches. M&As, Recent Developments
Competitive Landscape – Market Share Analysis, Market Leaders, Emerging Players, Vendor Benchmarking, Developmental Strategy Benchmarking, PESTLE Analysis, Value Chain Analysis
Company Profiles – Overview, Business Segments, Business Performance, Product Offering, Key Developmental Strategies, SWOT Analysis
Buy your copy here: https://www.globalinsightservices.com/checkout/single_user/GIS21593/
With Global Insight Services, you receive:
10-year forecast to help you make strategic decisions
In-depth segmentation which can be customized as per your requirements
Free consultation with lead analyst of the report
Infographic excel data pack, easy to analyze big data
Robust and transparent research methodology
Unmatched data quality and after sales service
Contact Us:
Global Insight Services LLC
16192, Coastal Highway, Lewes DE 19958
E-mail: [email protected]
Phone: +1-833-761-1700
Website: https://www.globalinsightservices.com/
0 notes
Text
Health innovation and the future of medicines development
The report titled “Healthcare Flexible Packaging Market” has recently been added by We Market Research to get a stronger and more effective business outlook. It provides an in-depth analysis of the different attributes of the industry, such as trends, policies, and customers operating in different geographies. Research analysts use quantitative as well as qualitative analytical techniques to provide users, business owners, and industry professionals with accurate and actionable data.
The report includes an executive summary, global economic outlook, and overview sections which provide a consistent analysis of the Healthcare Flexible Packaging market. Additionally, the report in the Market Overview section outlines PLC analysis and PESTLE analysis to provide a thorough analysis of the market. The overview section details Porter's five forces analysis which helps to reveal a possible scenario of the market by disclosing a competitive scenario with respect to the Healthcare Flexible Packaging Market.
Get a Sample Copy of Report, Click Here: https://wemarketresearch.com/reports/request-free-sample-pdf/healthcare-flexible-packaging-market/1387
Key companies profiled in this research study are:
Dätwyler Holding
Inc Bemis Company
Inc Catalent Pharma Solutions
WestRock, Aptar, Inc
Mondi
Coveris S.A
Becton, Dickinson & Company
Berry Global
CCL Industries
, Winpak Ltd
Gerresheimer
Huhtamäki Oyj
Sealed Air
Healthcare Flexible Packaging Market Segmentation:
By Material
Plastics
Paper
Bioplastics
Aluminium
By Product
Seals
High Barrier Films
Wraps
Lids & Labels
Pouches & Bags
Others
By End Use
Pharmaceutical Manufacturing
Medical Device Manufacturing
Implant Manufacturing
Contract Packaging
Others
The leading players of the Healthcare Flexible Packaging industry, their market share, product portfolio, company profiles are covered in this report. Key market players are analyzed on the basis of production volume, gross margin, market value, and price structure. The competitive market scenario among Healthcare Flexible Packaging players will help the industry aspirants in planning their strategies. The statistics presented in this report are an accurate and useful guide to shaping your business growth.
This research report also presents practical and practical case studies to help you get a clearer understanding of the topic. This research report has been prepared through industry analysis techniques and presented in a professional manner by including effective information graphics whenever necessary. It helps ensure business stability and rapid development to achieve notable remarks in the global Healthcare Flexible Packaging market.
Purchase a Copy of this Healthcare Flexible Packaging Market research report at@ https://wemarketresearch.com/purchase/healthcare-flexible-packaging-market/1387?license=single
Finally, Healthcare Flexible Packaging Market report is the believable source for gaining the Market research that will exponentially accelerate your business. The report provides locales, economic conditions, item values, benefits, limits, creations, supplies, requests, market development rates, and numbers, etc. Healthcare Flexible Packaging Industry Report Announces Additional New Task SWOT Examination, Speculation Achievement Investigation and Venture Return Investigation.
Report Customization Service:
We Market Research customizes the report according to your needs. This report can be personalized to suit your requirements. Get in touch with our sales team so you can get a report tailored to your needs.
About We Market Research:
WE MARKET RESEARCH is an established market analytics and research firm with a domain experience sprawling across different industries. We have been working on multi-county market studies right from our inception. Over the time, from our existence, we have gained laurels for our deep rooted market studies and insightful analysis of different markets.
Our strategic market analysis and capability to comprehend deep cultural, conceptual and social aspects of various tangled markets has helped us make a mark for ourselves in the industry. WE MARKET RESEARCH is a frontrunner in helping numerous companies; both regional and international to successfully achieve their business goals based on our in-depth market analysis. Moreover, we are also capable of devising market strategies that ensure guaranteed customer bases for our clients.
Contact Us:
Mr. Robbin Joseph
Corporate Sales, USA
We Market Research
USA: +1-724-618-3925
Websites: https://wemarketresearch.com/
Email: [email protected]
0 notes
Text
Ensuring Industrial Efficiency with Activated Carbon Granular Manufacturers Solutions - Angel Chemicals
We are key players in the production of a versatile adsorbent material widely used in various industries, Activated Carbon Granular Manufacturers including water purification, air filtration, food and beverage processing, pharmaceuticals, and environmental remediation.

Raw Material Sourcing: It is source raw materials from various carbonaceous sources, with coconut shells and coal being among the most commonly used. The choice of raw material depends on factors such as availability, cost-effectiveness, and the desired properties . Sustainable sourcing practices, such as using renewable raw materials and ensuring ethical supply chains, are increasingly important considerations for minimize environmental impact.
Quality Control: It is critical in ensure consistency and performance of the final product. It is implement stringent quality control measures at every stage of production, including raw material testing, process monitoring, and product testing. Quality parameters such as particle size distribution, adsorption capacity, hardness, and ash content are closely monitored to meet industry standards and customer requirements.
Product Range: We are offer a diverse range of products tailored to meet the specific needs of different applications. These may include standard-grade for general-purpose water and air treatment, speciality-grade carbon for specific contaminants such as heavy metals or volatile organic compounds (VOCs), and customized products with unique properties or particle sizes. It may also offer value-added services such as impregnation with specialty chemicals or custom packaging options.
Certifications and Compliance: There are many activated carbon granular manufacturers adhere to international standards and certifications to ensure the quality and safety of their products. Common certifications include NSF/ANSI Standard 61 for drinking water treatment, ISO 9001 for quality management systems, and ASTM standards for physical and chemical properties. Compliance with regulatory requirements, such as the European Union's REACH regulation or the U.S. Food and Drug Administration's (FDA) regulations, is also essential for manufacturers serving specific markets or applications.
Market Reach: It is distribute their products through various channels, including direct sales to end-users, distributors, and retailers, as well as contracts with industrial customers and original equipment . They may also export activated carbon to international markets to meet global demand. Strong market reach and distribution networks enable to reach a wide customer base and provide timely support and services.
Research and Development: Research and development (R&D) play a crucial role in Activated Carbon Granular Suppliers to drive innovation, improve product performance, and develop new applications. It is invest in R&D initiatives to explore advanced activation techniques, optimize production processes, and enhance the sustainability. Collaboration with research institutions, universities, and industry partners helps stay at the forefront of technology and market trends.
Address : Angel Chemicals Pvt. Ltd., Plot No. 374/2, Nr. Hanuman Mandir, GIDC, Makarpura, Baroda, Gujarat - 390010
Phone No : +91-9825512916,
Email Id : [email protected]
Url : https://www.angelchemindia.com/manufacturers/activated-carbon-granular.html
#Polyelectrolyte Manufacturers#Anionic Polyelectrolyte Manufacturers#Flocculant Chemicals Manufacturers#Cationic Polyelectrolyte Manufacturers#Polyelectrolyte Powder Manufacturers#Color Removal Chemicals Manufacturers
0 notes
Text
Pharmaceutical Product Development: A Deep Dive into USSF's Expertise
In the highly competitive pharmaceutical industry, product development plays a pivotal role in bringing new and effective drugs to the market. USSF, a prominent name in the field, leverages its expertise in pharmaceutical product development to transform groundbreaking research into viable commercial products. From the meticulous planning stage to regulatory compliance and market launch, USSF's comprehensive approach ensures success at every step.
Drug Research and Development Process: The Key to Effective Medicines
Before a pharmaceutical product reaches the shelves, it undergoes an intricate and rigorous process of drug research and development (R&D). USSF's dedicated team of scientists and researchers tirelessly work to identify promising drug candidates, conduct preclinical and clinical trials, and rigorously evaluate their safety and efficacy. Through cutting-edge technology and industry best practices, USSF accelerates the R&D process, making it more efficient and cost-effective.
Contract Manufacturing of Formulations: USSF's Commitment to Quality
As a leading player in the pharmaceutical industry, USSF understands the value of contract manufacturing for formulations. Partnering with USSF for contract manufacturing allows companies to focus on their core competencies while USSF takes care of formulation development, process optimization, manufacturing, and packaging. USSF's state-of-the-art facilities and adherence to Good Manufacturing Practices (GMP) ensure that all formulations meet the highest quality standards.
USSF: Combining Precision and Innovation
USSF's success lies in its ability to strike a delicate balance between precision and innovation in every aspect of pharmaceutical product development. The company's team of scientists, engineers, and regulatory experts work collaboratively to design and optimize formulations, address challenges, and anticipate industry trends. Their forward-thinking approach allows USSF to stay ahead of the curve, constantly improving processes and delivering superior pharmaceutical products to clients and patients.
Regulatory Compliance: USSF's Cornerstone
In the pharmaceutical industry, regulatory compliance is crucial to ensure patient safety and trust. USSF's rigorous adherence to global regulatory guidelines ensures that all their processes meet the highest standards, from research and development to contract manufacturing. This commitment to safety and compliance guarantees that USSF's products are trusted by healthcare professionals and patients alike.
USSF: A Partner for Success
In the competitive landscape of pharmaceutical product development, USSF stands out as a trusted partner. With their collaborative approach, commitment to quality, and ability to navigate complex regulatory environments, USSF helps companies accelerate their drug development timelines, reduce costs, and achieve commercial success. By collaborating with USSF, organizations can leverage their vast experience and expertise to bring effective and innovative medicines to the market, making a difference in patients' lives worldwide.
In conclusion, USSF's prowess in pharmaceutical product development, their understanding of the drug research and development process, and their expertise in contract manufacturing of formulations make them an invaluable partner for companies in the pharmaceutical industry. Through their commitment to quality, compliance, and innovation, USSF continues to drive advancements in the field and deliver transformative pharmaceutical solutions.
#contract manufacturing of formulations#custom formulation manufacturing#pharmaceutical product development#cgmp vaccine adjuvant development
0 notes
Text
Third Party Pharma Manufacturing Company In Punjab
In the ever-evolving pharmaceutical industry, third-party manufacturing has emerged as a popular business model. Punjab, known for its robust pharmaceutical infrastructure, offers numerous opportunities for entrepreneurs looking to venture into the field. Rowlinges Lifesciences, a leading third-party pharma manufacturing company in Punjab, stands out for its commitment to quality, innovation, and customer satisfaction. In this blog, we will explore the key aspects of Rowlinges Lifesciences and how it has become a trusted partner for businesses seeking reliable and efficient pharmaceutical manufacturing solutions.
Overview of Third-Party Pharma Manufacturing
Third-party pharma manufacturing, also known as contract manufacturing, involves outsourcing the production of pharmaceutical products to a specialized manufacturing company. This model allows businesses to focus on marketing, sales, and distribution while relying on the expertise and infrastructure of the manufacturing partner. Rowlinges Lifesciences, with its state-of-the-art manufacturing facilities and adherence to international quality standards, offers a seamless and cost-effective solution for companies looking to bring their pharmaceutical products to market.
Advantages of Choosing Rowlinges Lifesciences
Quality Assurance: Rowlinges Lifesciences prioritizes quality at every stage of the manufacturing process. Their manufacturing facilities are equipped with cutting-edge technology and adhere to stringent quality control measures, ensuring that the products meet the highest standards of efficacy and safety.
Regulatory Compliance: Rowlinges Lifesciences complies with all the necessary regulatory requirements, including Good Manufacturing Practices (GMP) and ISO certifications. This ensures that the products manufactured by Rowlinges Lifesciences are of superior quality and meet the regulatory standards set by authorities.
Customization and Flexibility: Rowlinges Lifesciences understands that each client has unique requirements. They offer a wide range of manufacturing capabilities, allowing for customization of formulations, packaging, and labeling. This flexibility enables businesses to create products that cater to specific market demands.
Timely Delivery: Rowlinges Lifesciences is known for its efficient supply chain management and timely delivery of products. Their streamlined processes and robust logistics ensure that clients receive their orders promptly, helping them meet market demands and maintain customer satisfaction.
Manufacturing Capabilities
Rowlinges Lifesciences boasts a comprehensive range of manufacturing capabilities, including tablets, capsules, syrups, injections, ointments, and more. Their manufacturing facilities are equipped with modern machinery and technology, enabling efficient production and ensuring consistent product quality. The company follows strict quality control protocols throughout the manufacturing process, from raw material sourcing to packaging and labeling.
Research and Development
Rowlinges Lifesciences places great emphasis on research and development (R&D) to stay at the forefront of pharmaceutical innovation. Their dedicated team of scientists and researchers continuously work on developing new formulations, improving existing products, and exploring novel drug delivery systems. This commitment to R&D enables Rowlinges Lifesciences to offer cutting-edge pharmaceutical solutions to their clients.
Quality Control and Assurance
Quality control is a top priority at Rowlinges Lifesciences. The company has a well-equipped quality control laboratory staffed by experienced professionals who conduct rigorous testing and analysis to ensure the highest quality standards. From raw material testing to in-process checks and final product analysis, every step is meticulously monitored to maintain product integrity and safety.
Regulatory Compliance
Rowlinges Lifesciences strictly adheres to all regulatory guidelines and standards. The company holds various certifications, including ISO 9001:2015, WHO-GMP, and Schedule M compliance. These certifications validate Rowlinges Lifesciences' commitment to quality, safety, and compliance with international standards.
Packaging and Labeling
Rowlinges Lifesciences understands the importance of attractive and informative packaging and labeling. They offer customized packaging solutions that not only protect the products but also enhance their market appeal. The company ensures that all packaging and labeling comply with regulatory requirements and provide accurate information to end-users.
Conclusion
Rowlinges Lifesciences, as a leading third-party pharma manufacturing company in Punjab, has established itself as a reliable and trusted partner for businesses seeking high-quality pharmaceutical manufacturing solutions. With their commitment to quality, regulatory compliance, and customer satisfaction, Rowlinges Lifesciences offers a comprehensive range of manufacturing capabilities, supported by robust research and development and stringent quality control measures. Choosing Rowlinges Lifesciences as a manufacturing partner ensures access to cutting-edge pharmaceutical solutions and a seamless manufacturing process. Partner with Rowlinges Lifesciences and unlock your business's potential in the pharmaceutical industry.
0 notes
Text

Third Party Manufacturing in Pharma: Nimbles Biotech Leading the Innovation Wave
In the dynamic landscape of pharmaceuticals, innovation and efficiency are paramount. As companies strive to meet the demands of the market while maintaining quality and compliance, the concept of third party manufacturing emerges as a beacon of opportunity. Today, we delve into the realm of third party manufacturing in pharma, exploring its nuances, benefits, and the transformative role it plays in the industry. Join us as we uncover the partnership between Nimbles Biotech, a pioneering franchise company, and its manufacturing arm, Philanto Wellness in Dera Bassi, Punjab, India.
Understanding Third Party Manufacturing in Pharma
Third party manufacturing, also known as contract manufacturing, involves outsourcing the production of pharmaceutical products to external manufacturers. This collaborative approach allows companies to leverage specialized facilities, expertise, and resources without the burden of owning and operating manufacturing units themselves. It offers a cost-effective solution, accelerates time-to-market, and enables companies to focus on their core competencies such as research, marketing, and distribution.
Philanto Wellness: Powering Excellence in Manufacturing
At the heart of Nimbles Biotech's manufacturing prowess lies Philanto Wellness, its state-of-the-art manufacturing unit located in Dera Bassi, Punjab, India. Equipped with cutting-edge technology, stringent quality controls, and a skilled workforce, Philanto Wellness exemplifies excellence in pharmaceutical manufacturing. From formulation development to packaging and distribution, every aspect is meticulously orchestrated to ensure adherence to global standards and regulatory requirements.
Benefits of Third Party Manufacturing with Nimbles Biotech
Partnering with Nimbles Biotech for third party manufacturing in Pharma offers a myriad of benefits for pharmaceutical companies:
Quality Assurance: With a relentless focus on quality, Nimbles Biotech ensures that every product manufactured meets the highest standards of safety, efficacy, and purity.
Cost Efficiency: By outsourcing manufacturing to Philanto Wellness, companies can significantly reduce overhead costs associated with setting up and maintaining manufacturing facilities.
Flexibility and Scalability: Nimbles Biotech offers flexible manufacturing solutions tailored to the specific needs and requirements of its partners. Whether scaling up production or adapting to market fluctuations, Nimbles Biotech provides agile solutions to drive growth.
Regulatory Compliance: Navigating the complex landscape of regulatory compliance can be daunting. Nimbles Biotech brings unparalleled expertise in regulatory affairs, ensuring compliance with local and international regulations.
Focus on Innovation: By outsourcing manufacturing responsibilities, companies can allocate resources towards innovation, research, and development, fostering a culture of continuous improvement and advancement.
Conclusion: Driving Innovation through Collaboration
In conclusion, third party manufacturing in pharma represents a strategic imperative for companies seeking to thrive in a competitive marketplace. With Nimbles Biotech as a trusted partner and Philanto Wellness as a centre of manufacturing excellence, companies can unlock new opportunities for growth, efficiency, and innovation. Together, we embark on a journey fueled by collaboration, integrity, and a shared commitment to advancing healthcare worldwide.
As the pharmaceutical landscape continues to evolve, the partnership between Nimbles Biotech and Philanto Wellness stands as a testament to the transformative power of collaboration. In the pursuit of excellence, we invite pharmaceutical companies to join us in redefining the future of healthcare through third party manufacturing.
#third party pharma manufacturing#pharma manufacturing#thirdpartymanufacturing#3rd party manufacturing#pharmamanufacturing
0 notes
Text
Cytotoxic Drugs Contract Manufacturing: An Overview

Cancer has become one of the leading causes of death around the world. While research and new treatment options have improved survival rates over the years, chemotherapy remains a fundamental pillar in the fight against cancer. Chemotherapy uses cytotoxic, or cell-killing, drugs to slow or stop the growth of cancer cells in the body. With the rise in cancer cases and a growing need for affordable, high-quality cancer treatment, the contract manufacturing of cytotoxic drugs has become an important part of the global healthcare industry.
The Growing Demand for Cytotoxic Drugs
Recent statistics show cancer rates are continuing to increase globally. In 2020 alone, there were an estimated 19.3 million new cancer cases diagnosed worldwide. While advancements have been made in surgery, radiation therapy, immunotherapy and targeted drug therapy, chemotherapy remains the go-to treatment option for many cancers. As more people are diagnosed with cancer each year, the demand for cytotoxic drugs is increasing as well. Contract manufacturing helps meet this growing need by mass-producing these life-saving drugs.
Cytotoxic drugs are among the most difficult and complex to manufacture due to their inherent toxicity. Strict measures must be followed to ensure worker and environmental safety during production. Contract manufacturers specializing in cytotoxic drugs are equipped with advanced facilities and trained professionals to handle these drugs safely and efficiently on a large scale. Their specialized cytotoxic manufacturing capabilities help pharmaceutical companies quickly ramp up production capacity to keep up with rising cancer treatment demands.
Regulatory Compliance for Cytotoxic Manufacturing
Due to cytotoxic drugs' hazardous nature, the manufacturing process for these compounds must adhere to stringent regulatory standards and compliance protocols. Contract manufacturers take on full responsibility for navigating global cytotoxic drug regulations while partnering with biopharmaceutical clients. They are highly equipped to comply with quality control and assurance guidelines from regulatory bodies like the US FDA, EU EMA, WHO and more.
Manufacturing cytotoxic drugs requires sophisticated containment facilities, engineering controls, quality procedures and worker safety protocols. Leading contract service providers make extensive investments to develop world-class cytotoxic manufacturing infrastructure in compliance with current Good Manufacturing Practice (cGMP) regulations. Their single-use systems, isolator technologies and automated processes minimize environmental contamination and operator exposure risks. Comprehensive quality management systems also help ensure the identity, strength, purity and batch consistency of cGMP batches.
Key Cytotoxic Drug Manufacturing Capabilities
Contract service providers support biopharma partners across the entire cytotoxic drug development and commercial production lifecycle. Some of their core cytotoxic drug manufacturing capabilities include:
Active Pharmaceutical Ingredient (API) Manufacturing
- Synthesis of cytotoxic drug substances and intermediates via chemical, biotech and other specialized methods.
Fill/Finish Operations
- Filling drug products into vials and syringes under aseptic conditions for final packaging and labelling.
Analytical Testing and Characterization
- Comprehensive analytical testing of drug substances and products to confirm identity, strength, purity, quality and stability.
Stability Testing Programs
- Long-term stability testing of drug candidates and commercial batches to determine proper storage conditions and expiry dates.
Technology Transfer
- Technology transfer services to smoothly transition cytotoxic drug manufacturing processes from lab to commercial scale.
Commercial Production
- Large-scale cGMP manufacturing of cytotoxic APIs and drug products to meet global market demand.
0 notes
Text
The Vital Role of Pharmaceutical Contract Manufacturers

The journey of a life-saving drug from discovery to your local pharmacy shelf is a complex one. While pharmaceutical companies are household names for developing new drugs, they often rely on specialized partners to bring those drugs to fruition. This is where pharmaceutical contract manufacturers (CMOs) come in.
Who are CMOs?
CMOs are essentially manufacturing companies that specialize in producing pharmaceuticals on behalf of other companies. Think of them as the factories behind the scenes. Drug companies, often focused on research and development (RD), partner with CMOs to leverage their expertise and resources for large-scale production.
What do CMOs do?
There are two main types of CMOs:
Contract Manufacturing Organizations (CMOs): These companies handle the manufacturing of pre-formulated drugs. They receive the drug formula from the pharmaceutical company and take care of everything from sourcing raw materials to packaging the final product, ensuring strict adherence to quality and regulatory standards.
Contract Development and Manufacturing Organizations (CDMOs): These companies offer a wider range of services. They can assist with pre-formulation and formulation development, clinical trial manufacturing, and of course, large-scale commercial production.
Why are CMOs important?
CMOs play a critical role in the pharmaceutical industry for several reasons:
Reduced Costs: Building and maintaining their own manufacturing facilities can be expensive for pharmaceutical companies. Partnering with a CMO allows them to leverage existing infrastructure and expertise, leading to significant cost savings.
Faster Time to Market: CMOs have the experience and capacity to ramp up production quickly, allowing drugs to reach patients faster. This is especially crucial for life-saving medications.
Expertise and Flexibility: CMOs often specialize in specific types of drugs or dosage forms. This expertise ensures high-quality production and allows pharmaceutical companies to focus on their core competencies.
Compliance: The pharmaceutical industry is heavily regulated. CMOs maintain rigorous quality control procedures and ensure compliance with regulatory requirements like Good Manufacturing Practices (GMP).
The Future of Pharmaceutical Contract Manufacturing
The CMO industry is constantly evolving. As the demand for new and complex drugs increases, we can expect to see CMOs offering even more specialized services, such as gene therapy and biologic drug manufacturing. Additionally, with the globalized nature of the pharmaceutical industry, we can expect to see more CMOs expanding their operations internationally.
In conclusion, pharmaceutical contract manufacturers are a vital link in the drug development and production chain. By providing expertise, flexibility, and cost-effectiveness, CMOs play a critical role in bringing essential medications to patients around the world.
0 notes
Text
Pharmaceutical Packaging Market Dynamics: Navigating the Complex Regulatory Landscape

The pharmaceutical packaging market involves the packaging of various pharmaceutical products such as capsules and tablets, parenteral containers, blister packaging, closures and others. Packaging adds functionality, protection and aesthetic value to pharmaceutical products while also differentiating brands. The pharmaceutical packaging helps extend the shelf life of drugs, protect them from moisture and light and prevents microbial growth.
The global pharmaceutical packaging market is estimated to be valued at US$ 264.21 Bn in 2023 and is expected to exhibit a CAGR of 9.5% over the forecast period 2023 to 2030, as highlighted in a new report published by Coherent Market Insights.
Market Opportunity:
The growth of the pharmaceutical packaging market offers high growth opportunities for pharmaceutical packaging market. pharmaceutical packaging is a service that involves outsourcing of packaging operations to third-party providers. Pharmaceutical companies are increasingly relying on contract packagers to handle varied packaging requirements for their products. This is helping pharmaceutical companies reduce capital investments, focus resources on core areas of development and ensure packaging compliances. The contract packagers offer technical expertise, flexibility and economies of scale in packaging which is driving several pharmaceutical manufacturers to utilize their services. The rise in generic drugs market further supports outsourcing of packaging to contract service providers. The growth in pharmaceutical packaging is expected to drive significant demand for pharmaceutical packaging over the forecast period.
Porter’s Analysis
Threat of new entrants: The pharmaceutical packaging market requires high initial investments in machinery, manufacturing facilities and R&D which acts as a barrier for new players. Regulations of the pharmaceutical industry also poses challenges for new entrants.
Bargaining power of buyers: Buyers have moderate bargaining power due to the importance of pharmaceutical products and packaging for patient medication and safety. However, the presence of many players limits the buyer power.
Bargaining power of suppliers: The packaging material and machinery suppliers have moderate bargaining power due to the availability of substitute materials and global supply chains.
Threat of new substitutes: Threat of substitutes is low as pharmaceutical packaging needs to meet strict quality and regulatory standards. No alternative ensures the same level of product protection and information transfer as packaging.
Competitive rivalry: The market is highly competitive due to the presence of many global and local players. Intense competition keeps pricing pressures and drives innovation in materials, machineries and sustainable solutions.
SWOT Analysis
Strength: The market has immense growth opportunities due to rising healthcare expenditure, increasing generics market and ambitious expansion plans of pharmaceutical companies. Customized solutions help strengthen customer relationships.
Weakness: Complex global regulations increase cost of compliance. Raw material price volatility impacts profitability.
Opportunity: Emerging markets offer lucrative prospects. Improving living standards boost healthcare spending. Advances in packaging technologies open doors for value-added offerings.
Threats: Economic slowdowns can negatively impact sales. Stringent environmental norms curb certain material usages.
Key Takeaways
The global Pharmaceutical Packaging Market is expected to witness high growth due to the rising healthcare spending worldwide and increasing demands of pharmaceutical products across regions. The global Pharmaceutical Packaging Market is estimated to be valued at US$ 264.21 Bn in 2023 and is expected to exhibit a CAGR of 9.5% over the forecast period 2023 to 2030.
Asia Pacific region dominated the global market in terms of size in 2023 owing to high population densities, rapidly improving healthcare infrastructure and rising living standards in countries like India and China. The Indian pharmaceutical packaging market is anticipated to grow at the fastest rate over the forecast period 2023-2030. The Indian pharmaceutical packaging market is poised to exhibit strong double digit growth through the forecast period supported by increasing domestic pharmaceutical production and exports from India. North America and Western Europe markets are mature yet steadily growing, while Latin America, Middle East and Africa offer lucrative prospects for future market expansions.
Key players operating in the Pharmaceutical Packaging Market are Amcor Limited, Berry Plastics Corporation, MeadWestvaco Corporation, Becton Dickinson and Company, Owens-Illinois Inc., West Pharmaceuticals Services Inc., Schott Pharmaceuticals Services Inc., RPC Group Plc and Graphic Packaging International Inc. These key players are focused on expanding their geographical footprint and enhancing their product portfolio through frequent product innovation. Their emphasis on sustainable products and solutions will also help strengthen their position in the highly competitive pharmaceutical packaging industry.
#Pharmaceutical Packaging Market#Pharmaceutical Packaging Market Trends#Pharmaceutical Packaging Market Growth
0 notes
Text
Vikas Ecotech Limited Secures Significant Orders Valued at INR 225 Million in Specialty Polymer Compounds
In an impressive development on December 8, 2023, Vikas Ecotech Limited, a key player in the realm of Specialty Polymers and Chemicals, announced a groundbreaking achievement. The company proudly unveiled contracts totaling approximately INR 225 Million in value for their specialty polymer compounds. This substantial achievement underscores Vikas Ecotech's dominance in the industry and its continuous efforts to innovate and expand its product portfolio.
These recent orders, specifically for specialty polymer compounds, are slated for fulfillment within the next 30-45 days, aiming for completion by February 15, 2024. Notably, these orders are projected to contribute nearly INR 1000 Million in revenue to the Specialty Polymer Compounds business vertical for the ongoing fiscal year. The company's strategic vision anticipates further order inflows during the upcoming seasonal export period from January to March 2024, aiming to bolster its revenue by tapping into these opportunities.
Vikas Ecotech's success can be attributed to its unwavering commitment to technical advancement and innovation. The company's technical team invested considerable time and resources to develop superior-grade specialty compounds. These compounds offer enhanced efficiency and custom-tailored solutions, presenting maximum efficacy coupled with an optimal cost-to-performance proposition for end-users. Notably, several of these developed compounds have been patented, positioning Vikas Ecotech at the forefront of technological advancements in the industry.
Furthermore, Vikas Ecotech's strategic expansion plans are well underway. Recently, the company completed the acquisition of a Plasticizer Manufacturing Business valued at approximately Rs 270 Million in an all-cash deal. This acquisition is projected to contribute an additional revenue of around INR 2000+ Million annually in the initial year. The company plans to further expand the production capacity at the newly acquired plant, solidifying its position in the market.
About Vikas Ecotech Limited:
Vikas Ecotech Ltd., headquartered in New Delhi, is a leading player in the domain of Specialty Polymers & Specialty Additives and Chemicals for industries spanning agriculture, infrastructure, packaging, electrical, footwear, pharmaceuticals, automotive, medical devices, and other consumer goods. Notably, Vikas Ecotech stands out as the sole manufacturer of Organotin (Heat Stabilizers for Vinyl applications) in India, boasting in-house R&D facilities. The company's innovative approach has expanded its product portfolio to include niche materials and consumer products beyond its raw material businesses.
The company's commitment to compliance and transparency is evident through its listing on both the BSE (Scrip Code: 530961) and NSE (Scrip Code: VIKASECO) stock exchanges.
0 notes
Text
How to Get GDP Certification in Denmark?

GDP Certification in Denmark
GDP certification in Denmark, is not mandatory, but companies that want to move prescription drugs and clinical devices inside the EU are recommended to gain it. The Danish Medicines Agency (DMA) is the competent authority for issuing GDP certificates in Denmark.
Corporations should first have their premises and structures inspected and accredited through the DMA to achieve GDP certification. The inspection covers all aspects of the corporation’s operations that apply to GDP, including garage, packaging, labelling, delivery, and distribution.
After the inspection, the DMA will trouble a GDP certificate if the organization is determined to comply with all applicable GDP requirements. The certificate is valid for three years and can be renewed upon expiration.
GDP certification is voluntary. However, it can be a precious advertising device, demonstrating a commitment to excellence and compliance with EU rules. It can also make it simpler to achieve contracts with pharmaceutical agencies and different clients that require GDP certification.
The Process of GDP Certification
As of July 1, 2019, the GDP certification procedure in Denmark has been modified. Previously, groups needed to follow a GDP certificate from the Danish Medicines Agency (DMA). Now, corporations can apply for a certificate from the Danish Health Authority (DHA).
The DHA is liable for issuing GDP certificates to corporations that meet the requirements outlined in the European Union’s (EU’s) Good Distribution Practices (GDP) recommendations. These tips ensure that pharmaceutical products are dealt with and stored well at some point in the distribution chain.
Companies must publish software to the DHA for a GDP certificate. The software must outline the commercial enterprise’s storage and distribution centres and list the goods to be stored and disbursed at the centres.
Once the software is received, a DHA inspector will go to the enterprise’s facilities to ensure they meet the requirements outlined in the EU GDP suggestions. If the centres are located to comply, the enterprise will be issued a certificate.
The certificate is valid for three years, and the business should reapply for certification.
The technique of GDP certification is vital for companies that distribute pharmaceutical merchandise in Denmark. By ensuring that these corporations meet the EU GDP hints, the DHA facilitates to ensure that the goods they distribute are safe and effective.
The Benefits of GDP Certification
GDP Certification in Denmark isn’t just a certificate; it’s an excellent control machine ensuring that merchandise exported from Denmark meets quality standards. The Danish authorities have strict necessities for GDP certification, which all companies exporting from Denmark should meet.
The benefits of GDP certification are many and sundry. However, three of the most critical benefits are:
1. Improved pleasant manipulation: GDP certification guarantees that products exported from Denmark meet certain standards. It is useful for Danish businesses and their clients because it guarantees that merchandise is exceptional and fit for purpose.
2. Enhanced recognition:- GDP certification can beautify the reputation of Danish organizations because it demonstrates their commitment to excellence. It can result in increased income and advanced patron pleasure.
3. Access to new markets:- GDP certification can open new markets for Danish groups. In a few instances, certification is a prerequisite for exporting to positive countries. This means that GDP-licensed corporations have an aggressive benefit over non-certified corporations.
Overall, GDP certification in Denmark has many blessings for both Danish groups and their clients. It is a first-rate management machine that complements Danish businesses’ popularity and gives them entry to new markets.
The Challenges of GDP Certification
The GDP certification system in Denmark may take a lot of work for agencies, as several necessities ought to be met to be compliant.
One of the most important demanding situations is the requirement for businesses to have a strong first-rate control device in the region. This gadget must be able to track and display all elements of the company’s operations and be capable of displaying compliance with GDP requirements. Another challenge is the requirement for organizations to have in-depth information on the Danish healthcare system. Includes expertise in the repayment gadget, how capsules are priced, and how patient care is introduced.
Lastly, corporations must be able to expose their adequate financial assets in the region to help their operations. Includes having enough coins reachable to cover surprising costs and having the capability to obtain financing if needed.
Meeting all of those demanding situations may be difficult. Still, agencies need to consider that the GDP certification technique is designed to guard sufferers and make sure that they get hold of great, feasible care. By assembling all the necessities, agencies can display that they’re committed to presenting the best quality of care.
Why Factocert for GDP Certification in Denmark
We provide the best GDP consultants in Denmark, Who are very knowledgeable and provide the best solution. And to know how to get GDP certification in Denmark . Kindly reach us at mailto:[email protected]. GDP Certification consultants work according to GDP standards and help organizations implement GDP certification in Denmark with proper documentation. For More Information Visit: GDP Certification in Denmark
Related link:
• ISO 9001 certification in Denmark
• ISO 14001certification in Denmark
• ISO 45001 certification in Denmark
• ISO 27001 certification in Denmark
• ISO 22000 certification in Denmark
0 notes
Text
Fill Finish Manufacturing Market by Manufacturers, Types, Regions and Applications Research Report Forecast to 2030
"Market Research on Fill Finish Manufacturing Market - Growth Rate, Market Share & Size" is the name research released by The Insight Partners and is now out for purchase. The business focuses on consulting and specializes in syndicated market research. The company is assisting Fill Finish Manufacturing market investors by providing both qualitative and quantitative data through this study.
Business Environment Analysis
This market research offers the study of a range of external factors impacting Fill Finish Manufacturing market players. These factors include economic, technological, and environmental considerations. Businesses can optimize their strategies as per these influences. Fill Finish Manufacturing market is driven by certain factors and there might be some hindrances ahead, this section takes you through all these factors. This chapter focuses on the following aspects-
Fill Finish Manufacturing market trends
Economic conditions
Consumer behavior analysis
Technological landscape
Covid-19 Impact on Business Ecosystem
The pandemic of covid-19 caused a slowdown in startup ecosystems and businesses throughout the globe were affected. Companies suffered from a lack of capital and funds. Shortage of supplies and dependency on global networks resulted in gaps in production. Many businesses come up with new contingency plans to ensure their survival.
The worldwide pandemic has caused considerable disruption to economies and enterprises, as well as several hitherto unheard-of issues. Nevertheless, the full extent of the pandemic's influence is still unknown, and further in-depth longitudinal research is needed to fully explore this matter. Consequently, the purpose of this section is to shed light on the difficulties and possibilities that may arise in the new normal while keeping the pandemic in mind.
Fill Finish Manufacturing Market Forecast
The market research study guides organizations on market economics by identifying current market size, revenue potential, and total market share. This further includes projections on future market size and share in the forecast period. The company needs to comprehend its clientele and the demand it creates to focus on a smaller selection of items. Through this chapter, market size assists businesses in estimating demand in specific marketplaces and comprehending projected patterns for the future.
Fill Finish Manufacturing Market Competition Analysis
Key companies in the Fill Finish Manufacturing market are- IMA Industria Macchine Automatiche SpA, Nipro Medical Europe NV, Maquinaria Industrial Dara SL, Groninger and Co GmbH, SGD SA, Optima Packaging Group Gmbh, NNE AS, Stevanato Group SpA, Syntegon Technology GmbH, West Pharmaceutical Services Inc, Gerresheimer AG, Schott AG, Becton Dickinson and Co.
This chapter provides information about both long-standing and recent Fill Finish Manufacturing market participants. Comprehending the competition facilitates a company's understanding of its market position. The study provides insights into opportunities and dangers facing Fill Finish Manufacturing industry participants through this chapter. Opportunities for market expansion in the Fill Finish Manufacturing sector may be found by contrasting the price and organic growth methods employed by major market players.
Fill Finish Manufacturing Market Segmentation
Based on Product of Fill Finish Manufacturing Market Research report:
Prefilled Syringes
Glass Vials/Plastic Vials
Cartridges
Others
Based on Modality of Fill Finish Manufacturing Market Research report:
Recombinant Proteins
Monoclonal Antibodies
Vaccines
Cell Therapies and Biological Therapies
Gene Therapies
Others
Based on End User of Fill Finish Manufacturing Market Research report:
Contract Manufacturing Organizations
Biopharmaceutical Companies
Others
Based on Regions:
North America (U.S., Canada, Mexico)
Europe (U.K., France, Germany, Spain, Italy, Central & Eastern Europe, CIS)
Asia Pacific (China, Japan, South Korea, ASEAN, India, Rest of Asia Pacific)
Latin America (Brazil, Rest of Latin America)
The Middle East and Africa (Turkey, GCC, Rest of the Middle East and Africa)
Rest of the World…
0 notes