#GMP Testing Service
Text
GMP Testing Service Market: Global Industry Analysis and Forecast 2023 – 2030
Global GMP Testing Service Market was valued at USD 1.17 Billion in 2021 and is expected to reach USD 1.88 Billion by the year 2028, at a CAGR of 6.97% .
Major factors contributing to the market expansion for GMP testing services include the pharmaceutical industry's explosive growth and the ongoing trend of drug and device development. The trend of biopharmaceutical companies developing more medications and devices is being driven by the increasing burden of chronic diseases, rising healthcare costs, and the impending patent expiration of blockbuster drugs. This is projected to propel the expansion of the studied industry. The launch of novel medications with cutting-edge features and strategic partnerships, as well as the growing R&D efforts of pharmaceutical companies, are anticipated to fuel the market's expansion.
Get Full PDF Sample Copy of Report: (Including Full TOC, List of Tables & Figures, Chart) @
https://introspectivemarketresearch.com/request/15848
Updated Version 2024 is available our Sample Report May Includes the:
Scope For 2024
Brief Introduction to the research report.
Table of Contents (Scope covered as a part of the study)
Top players in the market
Research framework (structure of the report)
Research methodology adopted by Worldwide Market Reports
Moreover, the report includes significant chapters such as Patent Analysis, Regulatory Framework, Technology Roadmap, BCG Matrix, Heat Map Analysis, Price Trend Analysis, and Investment Analysis which help to understand the market direction and movement in the current and upcoming years.
Leading players involved in the GMP Testing Service Market include:
Eurofins Scientific, PPD Inc., Microchem Laboratory, Sartorius AG, North American Science Associates Inc., Laboratory Corporation of America Holdings (Covance Inc.), Sotera Health (Nelson Laboratories LLC), Almac Group, Pace Analytical, Wuxi AppTec, Intertek Group PLC, Charles River Laboratories and Others major players.
If You Have Any Query GMP Testing Service Market Report, Visit:
https://introspectivemarketresearch.com/inquiry/15848
Segmentation of GMP Testing Service Market:
By Type
Product Validation Testing
Bioanalytical Services
Packaging
Shelf-Life Testing
Others
By End-User
Pharmaceutical & Biopharmaceutical Companies
Medical Devices Company
By Regions: -
North America (US, Canada, Mexico)
Eastern Europe (Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe)
Western Europe (Germany, UK, France, Netherlands, Italy, Russia, Spain, Rest of Western Europe)
Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New Zealand, Rest of APAC)
Middle East & Africa (Turkey, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa)
South America (Brazil, Argentina, Rest of SA)
What to Expect in Our Report?
(1) A complete section of the GMP Testing Service market report is dedicated for market dynamics, which include influence factors, market drivers, challenges, opportunities, and trends.
(2) Another broad section of the research study is reserved for regional analysis of the GMP Testing Service market where important regions and countries are assessed for their growth potential, consumption, market share, and other vital factors indicating their market growth.
(3) Players can use the competitive analysis provided in the report to build new strategies or fine-tune their existing ones to rise above market challenges and increase their share of the GMP Testing Service market.
(4) The report also discusses competitive situation and trends and sheds light on company expansions and merger and acquisition taking place in the GMP Testing Service market. Moreover, it brings to light the market concentration rate and market shares of top three and five players.
(5) Readers are provided with findings and conclusion of the research study provided in the GMP Testing Service Market report.
Our study encompasses major growth determinants and drivers, along with extensive segmentation areas. Through in-depth analysis of supply and sales channels, including upstream and downstream fundamentals, we present a complete market ecosystem.
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
Acquire This Reports: -
https://introspectivemarketresearch.com/checkout/?user=1&_sid=15848
About us:
Introspective Market Research (introspectivemarketresearch.com) is a visionary research consulting firm dedicated to assisting our clients to grow and have a successful impact on the market. Our team at IMR is ready to assist our clients to flourish their business by offering strategies to gain success and monopoly in their respective fields. We are a global market research company, that specializes in using big data and advanced analytics to show the bigger picture of the market trends. We help our clients to think differently and build better tomorrow for all of us. We are a technology-driven research company, we analyse extremely large sets of data to discover deeper insights and provide conclusive consulting. We not only provide intelligence solutions, but we help our clients in how they can achieve their goals.
Contact us:
Introspective Market Research
3001 S King Drive,
Chicago, Illinois
60616 USA
Ph no: +1-773-382-1047
Email: [email protected]
#GMP Testing Service#GMP Testing Service Market#GMP Testing Service Market Size#GMP Testing Service Market Share#GMP Testing Service Market Growth#GMP Testing Service Market Trend#GMP Testing Service Market segment#GMP Testing Service Market Opportunity#GMP Testing Service Market Analysis 2023
0 notes
Text
GMP Testing Service Market Growth, Trends, Covid-19 Impact
The GMP Testing Service Market was valued at USD 1,097.23 Million in 2020, and it is expected to reach USD 1,625.60 Million by 2026, registering a CAGR of 6.97% from 2021 to 2026.
The GMP testing services are relevant to the various manufacturers in food and beverages industry, cosmetics production, pharmaceutical drugs and medical devices industry. GMP testing services are a must when it comes to obtaining a license for initiating the production where mostly safety and quality is evaluated. For export-oriented businesses adhering to the GMPs of place of export are an obligation.
The report is based on a thorough examination of the whole worldwide GMP testing service market, as well as all of its sub-segments, using exhaustive classifications. With contributions from industry professionals across the value chain, in-depth research and assessment are developed from premium primary and secondary information sources.
Read More Info@: https://introspectivemarketresearch.com/reports/gmp-testing-service-market/
The Major Players in the GMP Testing Service Market Include:
· Eurofins Scientific
· PPD Inc.
· Microchem Laboratory
· Sartorius AG
· North American Science Associates Inc.
· Laboratory Corporation of America Holdings (Covance Inc.)
· Sotera Health (Nelson Laboratories LLC)
· Almac Group
· Pace Analytical
· Wuxi AppTec
· Intertek Group PLC
· Charles River Laboratories
Market has segmented the global GMP Testing Service market on the basis of type, application, and region:
The GMP Testing Service market is segmented by type and application. Growth between segments over the period 2022-2028 provides accurate calculations and forecasts of revenue by type and application in terms of volume and value. This analysis can help you expand your business by targeting eligible niches.
IMR presents a detailed study, and summation of data from multiple sources by an analysis of key parameters. Our report on the GMP Testing Service market covers the following areas:
· GMP Testing Service Industry Sizing Analysis
· GMP Testing Service Industry Forecast Analysis
· GMP Testing Service Market Industry Analysis
Download a Free Sample Copy of the Market Report: -
https://introspectivemarketresearch.com/request/15848
By Type:
· Product Validation Testing
· Bioanalytical Services
· Packaging
· And Shelf-Life Testing
· And Other Service Types
By Application:
· Pharmaceutical And Biopharmaceutical Companies
· Medical Devices Company
By Regional Outlook (Revenue, USD Billion, 2017 – 2028)
North America (U.S., Canada, Mexico)
Europe (Germany, U.K., France, Italy, Russia, Spain, Rest of Europe)
Asia-Pacific (China, India, Japan, Southeast Asia, Rest of APAC)
Middle East & Africa (GCC Countries, South Africa, Rest of MEA)
South America (Brazil, Argentina, Rest of South America)
Get Discount on This Report @:
https://introspectivemarketresearch.com/discount/15848
Covid-19 Impact and Recovery Analysis on Industry:
The COVID-19 pandemic has had devastating effects on several industry verticals globally. To constrain the number of cases and slow the coronavirus spread, various public health guidelines were implemented in different countries across the globe. COVID-19 protocols ranging from declaring national emergency states, enforcing stay-at-home orders, closing nonessential business operations and schools, banning public gatherings, imposing curfews, distributing digital passes, and allowing police to restrict citizen movements within a country, as well as closing international borders. With the growing vaccination rate, governments are uplifting the protocols to give a boost to the stagnant economy. Like other industries, GMP Testing Service Market have experienced slowdown the growth, however market is expected bounce back as restrictions are being lifted up by governments across the globe.
IMR offers a comprehensive overview of the market through the analysis of key parameters such as revenue, price, competition, and promotions, as well as the study, synthesis, and summarization of data from different sources. It analyzes the leading industry drivers and shows numerous market components. The information offered is thorough, dependable, and the result of a comprehensive primary and secondary study. IMR market research reports offer a comprehensive global market as well as an in-depth strategic sourcing methodology and analysis based on qualitative and quantitative research to anticipate market growth.
If You Have Any Query of GMP Testing Service Market Report, Visit:
https://introspectivemarketresearch.com/inquiry/15848
Reasons to Purchase the GMP Testing Service Market Report:
· The report includes a plethora of information such as market dynamics scenario and opportunities during the forecast period
· Segments and sub-segments include quantitative, qualitative, value (USD Million,) and volume (Units Million) data.
· Regional, sub-regional, and country level data includes the demand and supply forces along with their influence on the market.
· The competitive landscape comprises share of key players, new developments, and strategies in the last three years.
· Comprehensive companies offering products, relevant financial information, recent developments, SWOT analysis, and strategies by these players.
0 notes
Text
GMP Testing Service Market Growth
Global GMP Testing Service Market was valued at USD 1.17 Billion in 2021 and is expected to reach USD 1.88 Billion by the year 2028, at a CAGR of 6.97% .

The GMP testing services are relevant to the various manufacturers in food and beverages industry, cosmetics production, pharmaceutical drugs and medical devices industry. GMP testing services are a must when it comes to obtaining a license for initiating the production where mostly safety and quality is evaluated. For export-oriented businesses adhering to the GMPs of place of export are an obligation.
The report is based on a thorough examination of the whole worldwide GMP testing service market, as well as all of its sub-segments, using exhaustive classifications. With contributions from industry professionals across the value chain, in-depth research and assessment are developed from premium primary and secondary information sources.
#GMP Testing Service Market#GMP Testing Service Market Size#GMP Testing Service Market Share#GMP Testing Service Market Analysis
0 notes
Text
GMP Private Label Skin Care | GMP Skin Care
GMP rules and regulations address a wide range of concerns, including as record keeping, personnel, qualifications, sanitation, cleaning and equipment.
GMP refers to the good manufacturing practices regulations proclaimed by the US Food and Drug Administration under the authority of the Federal Food, Drug, and Cosmetic Act. These rules, which have the force of law, require that manufacturers, processors, and packagers of drugs, medical devices, some food, and blood take assertive steps to ensure that their products are absolutely safe, pure, and effective. These regulations help customers protect themselves from purchasing non-effective or less dangerous products.
GMP guidelines and regulations address various issues, including record keeping, personnel, qualifications, sanitation, cleaning, process validation, and equipment that can affect the safety and quality of a product visit us -
#gmp testing service market#best skin care companies#best skin care tips#best hair growth products#best hair oil#gmp platform#GMP
0 notes
Link

पुरुषों या महिलाओं की सेक्स समस्याओं को गुप्त रोग कहा जाता है. इस तरह की समस्याओं को स्वास्थ्य की देखभाल के जरिये स्वस्थ सेक्स जीवन बनाए रखने में मदद करने के लिए निम्न कदम उठाए जा सकते हैं. शराब सम्बंधित दिशानिर्देशों का कढ़ाई से पालन करना धूम्रपान छोड़ना तनाव से बचना अधिक आराम चिंताओं या समस्याओं के बारे में अपने साथी से बात करना आदि को अपनाकर इसे कम कर सकते हैं. लगभग 10 में से 1 पुरुष यौन समस्याओं का अनुभव करते हैं.
4 notes
·
View notes
Text
In-Depth Look at Itsuwa: A Decade of Excellence in Vaping Innovation

Company Overview
Since its inception in 2007, Itsuwa has carved out a prestigious niche as a leading electronic cigarette manufacturer. Under the visionary leadership of CEO Jasmin Guo, the company has become an indispensable OEM/ODM partner for renowned global brands, pushing the boundaries of vaping technology and service.
Excellence in Manufacturing
Itsuwa prides itself on adhering to the strictest GMP and ISO9001 standards. The facility is equipped with state-of-the-art testing labs and maintains an impressively low defect rate of under 0.3%, ensuring top-tier product safety and quality.
Product Innovation
From the well-loved VapeSoul to the VOOM and AWAK series, Itsuwa is not just a manufacturer but an innovator at heart. Over 25 dedicated engineers drive its R&D, tailoring products to enhance every aspect of the vaping experience, catering to both casual users and enthusiasts seeking robust mod systems.
Operational Excellence
Itsuwa is synonymous with operational efficiency, guaranteeing rapid product delivery within just seven days through trusted couriers. The flexibility in order size handling demonstrates their commitment to customer satisfaction, big or small.
Research and Development
The R&D team, powered by over 30 passionate engineers, continuously innovates to elevate vaping experiences while optimizing costs. Their goal is not just to keep pace but to set the pace in the ever-evolving vaping landscape.
Customer-Centric Approach
Itsuwa’s dedication to customer relations is evident in their quick response time and regular client interactions. By valuing each customer's journey, Itsuwa builds lasting partnerships based on trust and mutual growth.
Embracing the Future
With the vaping industry in constant flux, Itsuwa remains at the forefront, committed to innovation and quality. As they advance, they continue to be the preferred choice for businesses navigating the complex vape market.
For a deeper dive into Itsuwa’s innovative product lines and exemplary services, make sure to visit their website. Stay tuned to our blog for the latest updates and expert insights in the vaping world. Join us on this journey and let’s vape smartly and stylishly together!
Read the full article
0 notes
Text
AG Organica locating the top Indian third-party cosmetic Manufacturer & Wholesale Supplier
What is third-party cosmetic manufacturing?
Outsourcing the production of cosmetics to a specialized manufacturer—often referred to as a contract manufacturer or a third-party manufacturer—is known as third-party cosmetic manufacturing. Under this arrangement, a cosmetic firm or brand collaborates with a manufacturing facility that possesses the necessary knowledge, tools, and resources to create and manufacture cosmetic items on the company's behalf. Research and development of new items, product trials, sampling, testing (when available), manufacture, filling, packing, and occasionally branding and designing are the main services included in this process.
AG Organica is a leading cosmetic manufacturer and wholesale supplier based in Noida, India. They offer a wide range of organic and natural skincare, haircare, personal care, and colour cosmetic products. They have over 27 years of experience in manufacturing natural and organic products

AG Organica has ISO 22000:2018 and GMP certifications, allowing them to connect with global markets
Their state-of-the-art facilities have a production capacity to meet industry demands efficiently
They offer private label, white label, OEM, contract manufacturing and custom manufacturing services
AG Organica has a strong focus on R&D, using the latest technology and equipment to formulate innovative products
Their products are made with premium, organic ingredients and go through rigorous quality checks.They provide competitive pricing tailored to client needs and flexible payment terms
AG Organica specializes in manufacturing a diverse range of products including face washes, scrubs, toners, masks, serums, creams, hair oils, lipsticks, eyeliners and more. They welcome client visits to their facility to showcase their commitment to quality and transparency.
With their expertise, infrastructure and dedication to innovation, AG Organica has established itself as a trust.
Expertise and resources:

Experts with cutting-edge facilities, skilled, trained, and informed personnel, as well as the technical and commercial know-how to execute manufacturing with ease, are third-party manufacturers. They are also prepared to follow different quality guidelines and regulations, which may guarantee a smooth operation and support the production of stable, high-quality products.
Reliability:
Every day, we produce a variety of goods that are carried out by other manufacturers. Compared to an amateur or inexperienced product creator, their compositions and procedures are more trustworthy.
Cost-effective:
The services and options available to third-party manufacturers for materials, packaging, formulations, etc., may otherwise be more expensive and require more time. These resources allow consumers and brands that are just getting started or have limited funds to have a wide range of options without having to shell out a ton of cash.
Focus on brand launch:

Cosmetic brands can focus on their core competencies, such as marketing, branding, product placement, market knowledge, comparative analysis, etc., by outsourcing the production process to a third party. This enables them to devote greater resources to areas that have a direct bearing on their market presence and brand identity.
Ease and flexibility:
AG Organica Third-party manufacturers frequently have little trouble managing high production quantities or adapting to fluctuations in demand. Everything from bulk orders to minimum quantity orders, necessary components to customized products, can be handled by third-party producers. Their ability to handle production issues, market factors, and range requirements is remarkable.
Regulatory compliances:
AG Organica Reputable third-party manufacturers have extensive knowledge of testing, evaluations, paperwork, certificates, and other necessary needs. They can make it easier for people and brands to enter the market because of their knowledge and experience.
How to find an excellent third-party cosmetic manufacturer in India?
Everybody and every brand wants to work with the top third-party cosmetic manufacturer who can match their tastes and preferences and assist with bespoke manufacturing. It's easy to identify a reliable manufacturer! Just remember the following things:
Research:
To find possible Indian producers of cosmetics, do extensive research. Seek out manufacturers of cosmetics who have a solid track record in the field. Speak with them, get to know them, pay them a visit if you can, and make sure you have everything you need. A reputable manufacturer will be pleased to help you with your initial inquiries.
Evaluate their expertise
After you've narrowed down your list of possible manufacturers, assess each one's level of experience and knowledge regarding the particular category of cosmetics you wish to create. Take into account their experience, client testimonials, and any credentials they could possess
Quality assurance:

When it comes to cosmetics, quality is important. Verify whether the manufacturer has quality control procedures and adheres to good manufacturing practices (GMP). Check if they have industry certifications like ISO or GMP to make sure they adhere to requirements.
Customization options:
Find out if the company provides customized manufacturing if that's what you need. Examine their range of expertise and capacity to formulate, package, and label goods according to your unique needs.
Communication and responsiveness:
An effective cooperation requires effective communication. Evaluate how willing and responsive the manufacturer is to respond to your questions. A trustworthy and open manufacturer will answer your questions, keep lines of communication open, and address any concerns you may have.
Request samples:

Request samples of their products to assess the quality of their offerings before making a final decision. If necessary to fulfil your standard, resample.
Cost and minimum order quantity:
Talk to the manufacturer about the cost and the minimum order quantity (MOQ). Make sure their MOQ satisfies your production requirements and that their pricing is inside your budget.
Legal compliance:
Check to see if the producer abides by all laws and rules about the production of cosmetics in India. This entails following laws about product safety, labelling, and packaging.
Recall that establishing a successful collaboration for customized manufacturing includes conducting in-depth research, evaluating the experience and quality standards of potential third-party cosmetic manufacturers in India, and communicating effectively.
Conclusion: AG Organica is a leading manufacturer and supplier of pure & natural essential oils, carrier oils, and cosmetic products across the globe. They are a technology-driven company with a large R&D department, well-equipped laboratories, and talented technical officials.
AG Organica is the most promising contract manufacturer in India, serving partners with comprehensive product solutions including regulatory approvals & support, customized packaging, and more. They have a wide range of organic cosmetic products and also do private labeling & product customization on demand For More Information
Contact Us
Mob. +91 8929 440 683
Website: https://www.pureoilsindia.com/
#CosmeticManufacturer#CosmeticSupplier#CosmeticsBulkSupplier#CosmeticsWholesaleSupplier#CosmeticExporter#CosmeticBulkExporter#CosmeticsWholesaleExporter#PrivateLabelCosmeticsWholesaleExporter#PrivateLabelCosmeticsManufacturer#PrivateLabelCosmeticsSupplier#PrivateLabelCosmeticsExporter#PrivateLabelCosmeticsBulkExporter#OriginalEquipmentManufacturer#OriginalDesignManufacturer#PrivateLabeling#whiteLabeling
0 notes
Text
Exploring the Significance of Active Pharmaceutical Ingredients in Drug Development
Introduction:
Active Pharmaceutical Ingredients (APIs) serve as the cornerstone of medication efficacy, constituting the biologically active elements that drive therapeutic effects in pharmaceuticals. In this article, we'll explore the pivotal function of APIs throughout the drug development process and their crucial role in crafting medicines that are both safe and effective.

Understanding Active Pharmaceutical Ingredients:
APIs represent the active constituents of drugs responsible for eliciting therapeutic responses within the human body. By targeting specific biological processes or receptors, APIs effectively manage various medical conditions.
Formulation Development:
APIs provide the foundation for drug formulation, enabling pharmaceutical scientists to design delivery systems that optimize factors like bioavailability, stability, and drug release. The careful selection and characterization of APIs are essential in determining a drug's overall efficacy.
Safety and Efficacy:
Before integration into drug development, APIs undergo rigorous testing to ensure their safety and efficacy. Preclinical studies and clinical trials meticulously assess the pharmacokinetics, pharmacodynamics, and toxicological properties of APIs to ascertain their therapeutic potential and establish suitable dosage regimens.
Quality Control:
Maintaining stringent quality standards throughout API manufacturing is imperative to guarantee the safety and effectiveness of the final drug product. From raw material sourcing to synthesis, purification, and packaging, thorough quality control measures are enforced. Analytical techniques are utilized to verify the identity, purity, and potency of APIs.
Regulatory Compliance:
API manufacturing adheres to strict regulatory guidelines to uphold product quality, consistency, and patient safety. Regulatory bodies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) set forth guidelines and conduct inspections to ensure compliance with good manufacturing practices (GMP) for APIs.
Generic Medications:
APIs are instrumental in producing generic medications, which contain identical active ingredients to their branded counterparts. Demonstrating bioequivalence is crucial for generic drugs, ensuring they produce similar therapeutic effects. Therefore, maintaining API quality and consistency is paramount to the efficacy and safety of generic medications.
Innovations in API Development:
Ongoing advancements in API development have led to improved drug delivery systems, heightened bioavailability, and enhanced patient adherence. Innovations like nanoparticles, liposomes, and prodrugs enable targeted and sustained drug release, thereby maximizing the therapeutic potential of APIs.
Conclusion:
Active Pharmaceutical Ingredients (APIs) form the bedrock of drug development, playing an indispensable role in formulating safe and effective medications. Through extensive research, rigorous quality control measures, and regulatory compliance, APIs contribute to the creation of life-saving treatments that enhance the quality of life for millions worldwide. At Global Pharma Tek, our comprehensive Drug Development Services encompass the expertise needed to harness the potential of APIs in creating innovative therapies that address unmet medical needs and propel pharmaceutical science forward. As pharmaceutical science continues to progress, APIs will remain central to the discovery and development of groundbreaking therapies.
0 notes
Text
The Value of GLP Certification
What is GLP Certification?
Good Laboratory Practice is referred to as GLP. GLP Certification in Iraq In order to guarantee the quality and dependability of the data produced, GLP accreditation guarantees that laboratories doing clinical medical and environmental safety research adhere to standard operating procedures and standards. It is especially significant for sectors like chemicals, agrochemicals, and pharmaceuticals where regulatory licensing of products depends on precise and trustworthy data.
The process of obtaining GLP certification usually entails adhering to regulatory guidelines established by institutions like the FDA and the Office for Development and Economic Cooperation (OECD).
Benefits of GLP Certification?
GMP Implementation in Kenya offers several benefits to laboratories and organizations involved in non-clinical health and environmental safety studies:
Regulatory Compliance: GLP accreditation guarantees that labs adhere to standards established by agencies like the FDA and OECD. It is frequently necessary to adhere to these criteria in order to submit data to regulatory bodies for product approval.
Data Integrity: Generating precise, dependable, and repeatable data is crucial, and this is emphasized by GLP principles. GLP-certified labs raise the data's trustworthiness and integrity by following established protocols and quality control techniques.
Quality Assurance: In order to receive GLP accreditation, laboratories must set up strong quality assurance procedures to keep an eye on and manage every facet of the research process. This results in better data quality and more dependable study results by assisting in the identification and mitigation of potential mistakes or deviations.
How much does the GLP Certification cost?
GMP Cost in Zambia complexity and size of the the lab, the nature of certification (e.g., individual tests or the entire facility), the nation or region in which the certification is wanted, and the selected certification body or checking agency are just a few of the variables that can greatly affect the price of GLP certification. Moreover, costs might also cover things like regular compliance efforts, infrastructure improvements, training, and the creation of documentation.
The initial certification fees for a small to small institution pursuing GLP certification to a particular set of tests might vary from a few thousand to thousands of dollars. Costs may increase to the millions of dollars or more for larger labs, those pursuing certification for several tests, or facilities as a whole.
GLP Certification Audit process and implemention?
GMP Audit in Senegal In order to verify that a laboratory adheres to the best practice guidelines, an audit procedure known as the GLP certification audits consists of multiple parts. An outline of the common audit procedure and its steps of implementation is provided below:
Preparation: The laboratory should research GLP rules and recommendations relevant to their industry and location in order to fully prepare for the audit. They should also make sure that all required policies, guidelines, and quality control systems are current and in place.
Certification Body Selection: To do the GLP audit, the laboratory chooses a recognized certification organization or auditing firm. Selecting a reliable company with GLP certification experience is crucial.
Audit Planning: The laboratory and the certifying authority work together to organize the audit. This involves figuring out the audit's parameters, setting up the dates, and identifying the main players that will be involved in the process.
How to get the GLP consultant services?
GLP Consultants Services in philippines To obtain GLP consultant services for B2B certification, you can follow these steps:
Look Up and Select Consultants: Start by looking at GLP consultants who provide services specifically to companies looking to become certified. Seek out advisors who have worked in your sector and area and who understand the particular GLP regulations that apply to your kind of testing facility or laboratory.
Examine Credentials and Experience: Make sure prospective consultants have the necessary training and work experience. Seek out consultants with a history of helping clients obtain successful GLP certifications and who are accredited or certified by pertinent organizations. To evaluate the repute and dependability of the consultant, you can also look for references or customer endorsements.
Support for the Audit: In order to help with audit activities, respond to questions from the auditor, and make sure the audit goes well, the consultant may offer on-site support throughout the certification audit.
Post-Audit Follow-Up: The consultant should assist in resolving any non-conformities found during the audit and support the execution of remedial steps as necessary. They might also offer advice on how to continue adhering to GLP guidelines and how to get ready for upcoming audits or re-certification.
0 notes
Text
GMP Testing Service Market: Global Industry Analysis and Forecast 2023 – 2030
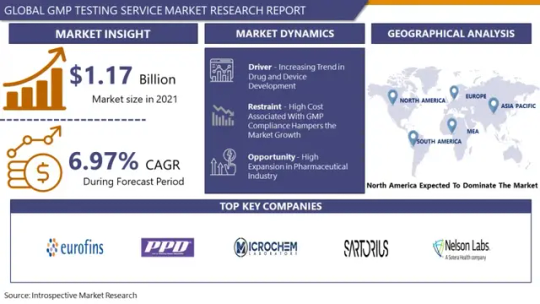
Global GMP Testing Service Market was valued at USD 1.17 Billion in 2021 and is expected to reach USD 1.88 Billion by the year 2028, at a CAGR of 6.97% .
Major factors contributing to the market expansion for GMP testing services include the pharmaceutical industry's explosive growth and the ongoing trend of drug and device development. The trend of biopharmaceutical companies developing more medications and devices is being driven by the increasing burden of chronic diseases, rising healthcare costs, and the impending patent expiration of blockbuster drugs. This is projected to propel the expansion of the studied industry. The launch of novel medications with cutting-edge features and strategic partnerships, as well as the growing R&D efforts of pharmaceutical companies, are anticipated to fuel the market's expansion.
Get Full PDF Sample Copy of Report: (Including Full TOC, List of Tables & Figures, Chart) @
https://introspectivemarketresearch.com/request/15848
Updated Version 2024 is available our Sample Report May Includes the:
Scope For 2024
Brief Introduction to the research report.
Table of Contents (Scope covered as a part of the study)
Top players in the market
Research framework (structure of the report)
Research methodology adopted by Worldwide Market Reports
Moreover, the report includes significant chapters such as Patent Analysis, Regulatory Framework, Technology Roadmap, BCG Matrix, Heat Map Analysis, Price Trend Analysis, and Investment Analysis which help to understand the market direction and movement in the current and upcoming years.
Leading players involved in the GMP Testing Service Market include:
Eurofins Scientific, PPD Inc., Microchem Laboratory, Sartorius AG, North American Science Associates Inc., Laboratory Corporation of America Holdings (Covance Inc.), Sotera Health (Nelson Laboratories LLC), Almac Group, Pace Analytical, Wuxi AppTec, Intertek Group PLC, Charles River Laboratories and Others major players.
If You Have Any Query GMP Testing Service Market Report, Visit:
https://introspectivemarketresearch.com/inquiry/15848
Segmentation of GMP Testing Service Market:
By Type
Product Validation Testing
Bioanalytical Services
Packaging
Shelf-Life Testing
Others
By End-User
Pharmaceutical & Biopharmaceutical Companies
Medical Devices Company
By Regions: -
North America (US, Canada, Mexico)
Eastern Europe (Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe)
Western Europe (Germany, UK, France, Netherlands, Italy, Russia, Spain, Rest of Western Europe)
Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New Zealand, Rest of APAC)
Middle East & Africa (Turkey, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa)
South America (Brazil, Argentina, Rest of SA)
What to Expect in Our Report?
(1) A complete section of the GMP Testing Service market report is dedicated for market dynamics, which include influence factors, market drivers, challenges, opportunities, and trends.
(2) Another broad section of the research study is reserved for regional analysis of the GMP Testing Service market where important regions and countries are assessed for their growth potential, consumption, market share, and other vital factors indicating their market growth.
(3) Players can use the competitive analysis provided in the report to build new strategies or fine-tune their existing ones to rise above market challenges and increase their share of the GMP Testing Service market.
(4) The report also discusses competitive situation and trends and sheds light on company expansions and merger and acquisition taking place in the GMP Testing Service market. Moreover, it brings to light the market concentration rate and market shares of top three and five players.
(5) Readers are provided with findings and conclusion of the research study provided in the GMP Testing Service Market report.
Our study encompasses major growth determinants and drivers, along with extensive segmentation areas. Through in-depth analysis of supply and sales channels, including upstream and downstream fundamentals, we present a complete market ecosystem.
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
Acquire This Reports: -
https://introspectivemarketresearch.com/checkout/?user=1&_sid=15848
About us:
Introspective Market Research (introspectivemarketresearch.com) is a visionary research consulting firm dedicated to assisting our clients to grow and have a successful impact on the market. Our team at IMR is ready to assist our clients to flourish their business by offering strategies to gain success and monopoly in their respective fields. We are a global market research company, that specializes in using big data and advanced analytics to show the bigger picture of the market trends. We help our clients to think differently and build better tomorrow for all of us. We are a technology-driven research company, we analyse extremely large sets of data to discover deeper insights and provide conclusive consulting. We not only provide intelligence solutions, but we help our clients in how they can achieve their goals.
Contact us:
Introspective Market Research
3001 S King Drive,
Chicago, Illinois
60616 USA
Ph no: +1-773-382-1047
Email: [email protected]
#GMP Testing Service#GMP Testing Service Market#GMP Testing Service Market Size#GMP Testing Service Market Share#GMP Testing Service Market Growth#GMP Testing Service Market Trend#GMP Testing Service Market segment#GMP Testing Service Market Opportunity#GMP Testing Service Market Analysis 2023
0 notes
Text
https://www.maximizemarketresearch.com/market-report/gmp-testing-service-market/188536/
0 notes
Text
GMP and Quality Control Integration in Pharmaceutical Companies
What is GMP Certification?
GMP Certification in Chad certification is a system that ensures products are consistently produced and controlled according to quality standards. It is designed to minimize the risks involved in any pharmaceutical production that cannot be eliminated through testing the final product. Key elements include stringent hygiene and sanitation protocols, proper facility and equipment maintenance, comprehensive staff training, detailed documentation, and regular inspections. GMP certification is crucial for industries like pharmaceuticals, food and beverages, cosmetics, and medical devices, ensuring products meet regulatory requirements and building consumer trust.
How business will get Benefits by GMP Certification?
GMP Implementation in Nigeria ensures products are consistently produced and controlled according to high quality standards. Reduces the risk of contamination, mix-ups, and errors in the production process. It helps businesses comply with national and international regulations, which is often a legal requirement for selling products in certain markets. Reduces the risk of regulatory fines, recalls, and legal actions due to non-compliance.
What kind of Business is suitable for GMP certification?
Drug Manufacturers Ensures that medications are safe, effective, and free from contamination.Biotech Companies Maintains high standards in the production of biological products and therapies.GMP Audit in Zimbabwe business involved in the production of consumable goods, healthcare products, or items that come into contact with the human body can benefit from GMP certification. This certification helps ensure product quality, safety, and regulatory compliance, which are crucial for protecting consumers and maintaining a good business reputation.
How much does GMP certification will cost ?
Larger companies with more complex operations and multiple sites will generally face higher costs than smaller, single-site businesses.The more employees involved in the processes that need to be audited, the higher the cost. GMP Cost in Mumbai businesses hire consultants to help them prepare for GMP certification. These fees can vary based on the consultant's experience and the extent of the services provided.
How To Get a GMP consultant ?
GMP Consultants Services in Jordan as a B2BCert consultant specializing in GMP Certification services, we can guide you through the process step-by-step to ensure a smooth and successful certification journey. Here's how we can assist you in obtaining GMP Certification Clearly define the scope of the project, including the specific areas where you need assistance (e.g., documentation, training, process improvements).Ensure the consultant has experience in your specific industry (e.g., pharmaceuticals, food and beverages, cosmetics).Professional Associations: Consult with groups like the Institute of Food Technologists (IFT) or the International Society for Pharmaceutical Engineering (ISPE) to find consultants. Make use of online resources like LinkedIn, industry-specific discussion boards, and directories for consultants. Consult suppliers, colleagues in the business, or professional networks for recommendations.
0 notes
Text
Lung Clear Pro™️ | CA Official Website | Get 85% Off Today
Lung Clear Pro is a new supplement made to help keep your lungs healthy. It's different from other remedies because it targets the main reason for breathing problems: trapped mucus.
It's made to help you sleep better, breathe deeper, stop coughing, and get back to living your life without breathing struggles. The special mix of natural ingredients is meant to clear out toxins from your lungs, get rid of stuck mucus, and improve the oxygen levels in your blood—all without leaving your home.
Get 85% Off Today Grab The Deal Now
Visit Official Website- Lung Clear Pro

Why Choose Lung Clear Pro?

GMP Certified
This product is certified under Good Manufacturing Practice standards.

100% Natural
We are proud to say that Lung Clear Pro is all natural, non-GMO and gluten free with additives.

FDA
Lung Clear Pro is manufactured in an FDA registered facility that meets strict FDA regulations.

Made In USA
Lung Clear Pro nutritional supplement is manufactured in a factory in the US.
What is Lung Clear Pro Dropper?
At Lung Clear Pro CA, we're a group of health lovers who want to help regular folks like you stay healthy and active. We work hard to create great products that support your energy and vitality, no matter how old you are.
All our stuff is made in the USA in a special facility that follows really strict rules to make sure everything is good for you. We test our products a lot to make sure they're really pure and top-quality.
We're not like other health companies that just want to make money quickly. We care about keeping you happy and coming back for more by giving you only the best stuff. You can trust that what you're getting from us is really good.
And here's the best part: if you try any of our products and they're not right for you, just call our customer service team in the CA, and we'll give you your money back, no questions asked. We're that confident in what we offer!
Lung Clear Pro CA Supplement Benefits
Better Breathing: Lung Clear Pro clears out mucus from your lungs, making it easier to breathe and improving airflow.
Respiratory Support: The special ingredients like Mullein Leaf, Cordyceps, Bromelain, Ginger, and Lemon Peel work together to support your lungs in different ways. They help with clearing mucus, reducing inflammation, and providing antioxidants, which all help keep your respiratory system healthy.
Reduced Inflammation: Lung Clear Pro contains ingredients like Bromelain and Ginger that help decrease inflammation in your lungs, making breathing easier and more comfortable.
Boosted Immune System: The antioxidants in Lemon Peel help support your immune system, which can protect your respiratory health by fighting off harmful substances.
Stress Response: Cordyceps helps your body handle stress better, which can have a positive impact on your respiratory health.
Overall Respiratory Wellness: Lung Clear Pro CA isn't just about relieving symptoms – it aims to improve your overall respiratory health by addressing congestion, inflammation, and supporting your immune system.
Natural and Safe: Lung Clear Pro is made from natural ingredients and doesn't contain any harmful chemicals, so it's safe to use and gentle on your body.
How Does Lung Clear Pro Work?
Lung Clear Pro is different from usual solutions like inhalers and oxygen tanks because it focuses on fixing the main problem behind breathing issues – trapped mucus in the lungs. Instead of just giving temporary relief, this supplement works to clear out that mucus for long-lasting results and better lung function.
The ingredients in Lung Clear Pro, like Mullein Leaf, Cordyceps, Bromelain, Ginger, and Lemon Peel, are all chosen because they're known to help with lung health. Together, they work to support your respiratory system in a natural way, targeting the root cause of breathing problems.
Safety and effectiveness are really important with Lung Clear Pro CA. It's made from 100% natural ingredients, so it's gentle but still powerful for your lungs. Plus, everything is grown and made in the USA in a really good facility that follows strict quality standards.
Whether you've been a smoker for a long time, deal with breathing issues, or just want to keep your lungs healthy, Lung Clear Pro tonic is an easy and safe option. And if you're not happy with it, there's a 180-day money-back guarantee, so you can try it risk-free. It's a simple way to take care of your lungs and improve your overall respiratory health.
Lung Clear Pro CA Customers Reviews

"After encountering dense smoke due to summer fires in my region, I decided to try Lung Clear Pro. This product truly lived up to its reputation in the Lung Clear Pro reviews. It provided remarkable relief, clearing my airways and making breathing much easier."

"I was skeptical, but I tried Lung Clear Pro CA," the user admitted. "I use an inhaler a lot because I've been smoking for a long time. After about two weeks," they continued, "my breathing got a lot better, and I hardly need my inhaler anymore. I've been coughing up a lot of mucus, and my chest feels clearer and lighter now."

"The results were astonishing! I haven't breathed this easily in years," exclaimed the user. "Normally, I spend hours coughing to clear my lungs. But after trying this product," they continued, "I feel ready for the day, breathing deeply and feeling happy."
Is Lung Clear Pro Safe And Effective?
Lungs Clear Pro guarantees both safety and efficacy. Our formula is meticulously composed using 100% natural ingredients, selected for their safety and potency, ensuring your well-being is our top priority. Free from harmful chemicals or additives, our supplement undergoes manufacturing in facilities adhering strictly to Good Manufacturing Practices (GMP) and FDA approval, ensuring utmost safety and purity throughout production.
Scientifically formulated, Lungs Clear Pro CA provides gentle yet robust support for your respiratory system. Each ingredient is carefully selected to work harmoniously, promoting optimal lung health without any adverse effects. Countless satisfied customers have reported improvements in breathing, better sleep, and an overall enhancement in quality of life after using Lungs Clear Pro.
Backed by a 180-day money-back guarantee, Lungs Clear Pro supplement offers a risk-free solution for your lung health. Your safety and satisfaction remain our primary commitments.
Natural Ingredients Of Lung Clear Pro
1.Cordyceps- Renowned for its adaptogenic properties, Cordyceps enhances your body's ability to manage stress. In Lung Clear Pro, it contributes significantly to respiratory support by potentially boosting oxygen intake, thereby improving lung health.
2. Bromelain- Extracted from pineapples, Bromelain is an enzyme with potent anti-inflammatory abilities. By alleviating inflammation in the lungs, it offers relief from respiratory discomfort and facilitates easier breathing.
3. Ginger- Celebrated for its anti-inflammatory and antioxidant characteristics, Ginger aids lung health by reducing airway inflammation. Its antioxidant properties shield the lungs from oxidative stress, ensuring optimal functionality.
4. Lemon Peel- Abundant in antioxidants, particularly vitamin C, Lemon Peel bolsters the immune system and sustains respiratory well-being. In Lung Clear Pro, it fortifies the defense against free radicals, safeguarding the lungs from potential damage.
5. Mullein Leaf- Recognized for its efficacy in clearing mucus from the respiratory tract, Mullein Leaf promotes easier breathing by addressing congestion and inflammation. It offers lasting relief and improves airflow, enhancing respiratory comfort.
Lung Clear Pro Offers 180 Money Back Policy
With Lung Clear Pro, you're covered by a 180-Day, 100% Money Back Guarantee. This means if you decide to change your mind within the next 180 days, just reach out to our Customer Service team in the CA, and we'll give you a refund.

No questions asked! Plus, you can keep the droppers as a thank you for trying Lung Clear Pro.
Lung Clear Pro Frequently Asked Questions?
Is Lung Clear Pro safe to use?
Lung Clear Pro is made from 100% natural and safe ingredients, so it's completely safe, effective, and natural. Many people use it every day without any problems, and there have been no reported side effects. It's made in the USA at our FDA-approved, GMP-certified facility, so it meets the highest standards. Plus, it's all-natural, vegetarian, and non-GMO. Just to be safe, talk to your doctor before using it if you have any medical conditions.
What If Lungs Clear Pro Doesn't Work For Me?
We're really sure our product will help you, so every bottle comes with our own 180-day 100% money-back guarantee. If you're not happy with your results for any reason, you can send back the unused portion and get a full refund, no questions asked.
How many bottles should I buy?
For the best results, use Lung Clear Pro for 3 to 5 months. Buying 3 to 6 bottles gets you discounts and is the minimum needed to see results. Remember, this discount isn't always available, so grab it while you can.
0 notes
Text
0 notes
Text
Understanding the Importance of GMP Certification in Eswatini

GMP (Good Manufacturing Practices) certification is a crucial standard for ensuring the quality, safety, and efficacy of pharmaceuticals, food products, and other goods in Eswatini. As a landlocked country in Southern Africa, Eswatini faces unique challenges and opportunities in its manufacturing and regulatory landscape.
GMP certification in Eswatini is essential for businesses operating in sectors such as pharmaceuticals, food and beverages, cosmetics, and medical devices. It signifies compliance with internationally recognized standards set forth by regulatory bodies like the World Health Organization (WHO) and local authorities. Achieving GMP certification demonstrates a company's commitment to producing goods of high quality and safety, meeting customer expectations, and adhering to legal requirements.
GMP Implementation in Eswatini
Understanding Regulatory Requirements: Begin by familiarizing yourself with the specific GMP regulations and guidelines applicable in Eswatini. These regulations are typically set by government agencies such as the Eswatini Medicines Regulatory Authority (EMRA) for pharmaceuticals or the Eswatini Standards Authority (SWASA) for food and beverages.
GMP Training: Train your employees on GMP principles, practices, and regulations. This includes training on hygiene, sanitation, good documentation practices, equipment calibration, and quality control measures.
Facility Design and Maintenance: GMP Implementation in seychelles - Ensure that your manufacturing facilities are designed and maintained according to GMP requirements. This includes proper layout, equipment maintenance, sanitation protocols, and environmental controls to prevent contamination.
Quality Management Systems (QMS): Implement a robust Quality Management System that encompasses GMP requirements. This includes procedures for batch records, product testing, quality assurance, change control, and risk management.
GMP Services in Eswatini
Consulting Services: Experienced GMP consultants provide guidance and support to businesses in understanding GMP requirements, conducting gap analysis, developing implementation strategies, and preparing for GMP certification audits.
Training and Workshops: GMP training programs and workshops are offered to educate personnel at all levels within an organization on GMP principles, regulations, best practices, and the importance of quality management systems.
Quality Management Systems (QMS) Development: Consultants assist in designing, implementing, and optimizing Quality Management Systems tailored to meet GMP standards. This includes document management, record keeping, change control, risk management, and corrective/preventive action processes.
GMP Audits and Inspections: GMP auditors conduct comprehensive audits and inspections of manufacturing facilities, processes, documentation, and quality control systems to assess compliance with GMP guidelines and identify areas for improvement.
Validation and Qualification Services: GMP Services in Botswana - Assistance is provided in validating and qualifying equipment, processes, and systems to ensure they meet GMP requirements and produce consistent and reliable results.
GMP Audit in Eswatini
Pre-Audit Preparation: Prior to the audit, the auditing team reviews relevant GMP regulations, standards, and guidelines applicable in Eswatini, such as those set by the Eswatini Medicines Regulatory Authority (EMRA) or the Eswatini Standards Authority (SWASA). The audit scope, objectives, and criteria are defined, and an audit plan is developed.
Audit Team Selection: Qualified auditors, either internal or external, are selected based on their expertise in GMP regulations, industry practices, and auditing methodologies. The audit team may include experts in areas such as quality assurance, quality control, production, documentation, and regulatory affairs.
On-Site Audit: The audit team conducts an on-site inspection of the manufacturing facility, including production areas, storage facilities, laboratories, quality control areas, and documentation centers. They evaluate equipment, processes, cleanliness, hygiene practices, personnel training, and compliance with GMP principles.
Documentation Review: Auditors review GMP-related documentation such as Standard Operating Procedures (SOPs), batch records, validation documents, quality manuals, training records, change control records, and regulatory submissions. They assess the completeness, accuracy, and adherence to GMP requirements.
How can I become accredited in Eswatini to practice GMP?
B2Bcert Consultants can be a great option if you're looking for GMP Certification Consultants in Eswatini to make sure that international regulations are followed and business procedures are improved. For the following reasons, picking B2Bcert as your GMP Certification Consultants in Eswatini makes sense. We take great pride in serving our clients, especially when we are able to offer them high-quality, reasonably priced care. A lot of individuals worry a lot about the budget at work. Unlike its competitors, B2Bcert provides solutions at reasonable costs without sacrificing the quality of its advisory services.
0 notes