#Cardiac Monitor Manufacturers
Text
Cardiac Monitor Manufacturers
A comprehensive range of cardiac monitors, as well as a variety of related products and services on the Hospital Product Directory platform. To help you refine the results for cardiac monitor manufacturers, Cardiac Monitor suppliers, and Cardiac Monitor dealers in India, Visit us for more details. B2B healthcare directory of hospital and healthcare product in india.pick from 2000+ product served by 10000+ suppliers.

0 notes
Text

At HospitalProductDirectory.com, we are providing the list of Cardiac Monitor Manufacturers & Cardiac Monitor Suppliers from India with a variety of related health care products and services on Hospital Product Directory.
1 note
·
View note
Text
0 notes
Text
Choosing the Right LifePak AED: A Comprehensive Guide
When selecting an automated external defibrillator (AED) for your business or organization, making an informed decision is crucial. LifePak stands out as a trusted and reliable choice among the top AED brands available in the market. This comprehensive guide will walk you through the factors to consider when choosing the right LifePak AED for your specific needs.

Assess Your Requirements:
Before delving into the details of LifePak AED models, assess your requirements. Consider the following factors:
a. Environment: Determine the environment in which the AED will be used. Is it for a workplace, school, gym, or public space? Different environments may have specific regulations or requirements.
b. User Experience: Evaluate the skill level of potential users. Are they trained, medical professionals or laypersons? AED models may vary in terms of ease of use and available features.
c. Budget: Determine your budget range. LifePak offers a variety of AED models with different price points, so it's essential to find a balance between your requirements and budget.
LifePak AED Models:
LifePak offers a range of AED models, each designed to cater to specific needs. Let's explore some of the popular models:
a. LifePak CR2: The LifePak CR2 is a user-friendly AED suitable for professional rescuers and laypersons. It features clear visual instructions and real-time CPR feedback to guide users effectively.
b. LifePak 1000: The LifePak 1000 is designed for professional rescuers and provides advanced monitoring and customization options. It offers flexibility in adapting to different rescue scenarios.
c. LifePak CR Plus: The LifePak CR Plus is a versatile AED suitable for various environments. It offers simple operation and is equipped with escalating energy levels to maximize the chances of a successful rescue.
Key Features and Considerations:
When comparing LifePak AED models, consider the following key features and factors:
a. CPR Feedback: Look for AED models that provide real-time CPR feedback, as this can significantly assist rescuers in performing effective chest compressions and rescue breaths.
b. ECG Monitoring: Some LifePak models offer ECG monitoring capabilities, allowing for more advanced cardiac monitoring during a rescue.
c. Energy Levels: Check the available energy levels of the AED models. Higher energy levels may be beneficial for specific cardiac arrest scenarios.
d. Battery Life and Maintenance: Consider the AED model's battery life and maintenance requirements. Longer battery life and low maintenance requirements can be advantageous, especially for busy environments.
e. Accessories and Compatibility: Ensure that the AED model you choose has readily available accessories such as electrode pads and batteries. Compatibility with standard accessories can simplify the maintenance process.
Training and Support:
Proper training and ongoing support are vital for effective AED implementation. LifePak offers training programs and resources to ensure users have the necessary skills and knowledge to operate the AED effectively. Consider the availability of training programs and the level of support the manufacturer or authorized distributors provide.
Conclusion:
Selecting the right LifePak AED involves carefully assessing your requirements, understanding the available models and their features, and considering factors such as budget, user experience, and maintenance. Following this comprehensive guide, you can make an informed decision and choose a LifePak AED that aligns with your needs. Remember, investing in a reliable AED is crucial to safeguarding lives and increasing the chances of successful resuscitation in a cardiac emergency.
0 notes
Text
Cardiac Diabetic Products Manufacturer
Cardiac Diabetic Medicine is a leading manufacturer of specialized products catering to individuals with both cardiac and diabetic conditions. Their innovative range includes medications, monitoring devices, and dietary supplements designed to manage and improve the health of those living with these interconnected health concerns, prioritizing holistic wellness solutions.

0 notes
Text
Sorin Stockert 3T Heater-Cooling System manufacturer & supplier in India: Octopus Med Pvt Ltd.
In the realm of medical technology, innovations play a pivotal role in enhancing patient care and outcomes. Among the many advancements, the Sorin Stockert 3T Heater-Cooler System stands out as a crucial component in cardiac surgeries, ensuring optimal temperature management during procedures. As a trusted manufacturer and supplier of Sorin Stockert 3T Heater-Cooler System in India, Octopus Med brings you the Sorin Stockert 3T Heater-Cooler System, delivering unparalleled performance and reliability.
About the Sorin Stockert 3T Heater-Cooler System
The Sorin Stockert 3T Heater-Cooler System is a sophisticated medical device designed to regulate the temperature of patient's blood and organs during cardiac surgeries. It consists of advanced heating and cooling components that maintain precise temperatures, critical for the success of complex procedures. Octopus Med offers the Sorin 3T Heater Cooler Systems, recognized for their superior quality and performance in the medical community.
Key Features of Sorin Stockert 3T Heater-Cooler System:
Precision Temperature Control: The Sorin Stockert 3T Heater-Cooler System ensures precise temperature management, allowing surgeons to maintain optimal conditions throughout the surgical procedure.
Reliable Performance: With its robust design and advanced technology, the Sorin 3T Heater Cooler guarantees reliable performance, contributing to seamless surgical experiences and positive patient outcomes.
Enhanced Safety Features: Sorin Stockert 3T Heater-Cooler Systems are equipped with enhanced safety features to mitigate risks and ensure patient safety during cardiac surgeries.
User-Friendly Interface: The intuitive interface of the Sorin Stockert 3T Heater-Cooler System facilitates ease of use for healthcare professionals, enabling efficient operation and monitoring during procedures.
Buy SORIN Stockert 3T at Best Price in India
Octopus Med offers the Sorin Stockert 3T Heater-Cooler System at the best price in India, making advanced medical technology accessible to healthcare facilities nationwide. Our commitment to affordability and quality ensures that healthcare providers can procure essential equipment without compromising on performance or budget.
Why Choose Octopus Med for Sorin Stockert 3T Heater-Cooler System?
Quality Assurance: Octopus Med is dedicated to delivering products of the highest quality, backed by rigorous quality assurance processes and adherence to international standards.
Expertise and Experience: With years of experience in the healthcare industry, Octopus Med has established itself as a trusted name, offering innovative solutions and unparalleled customer service.
Customer Satisfaction: At Octopus Med, customer satisfaction is paramount. We strive to exceed expectations by providing timely support, seamless transactions, and personalized service to our clients.
Nationwide Reach: Octopus Med's extensive distribution network ensures prompt delivery of Sorin Stockert 3T Heater-Cooler Systems to healthcare facilities across India, enabling timely access to essential medical equipment.
In conclusion, the Sorin Stockert 3T Heater-Cooler System from Octopus Med India represents a paradigm shift in cardiac surgery technology, empowering healthcare providers to deliver superior patient care. With its advanced features, reliability, and affordability, the Sorin Stockert 3T Heater-Cooler System is a valuable addition to any cardiac surgery suite. Contact Octopus Med today to buy SORIN Stockert 3T at the best price in India and elevate your surgical capabilities to new heights.
#heater cooler systems for patients#Buy Heater Cooler For Heart Lung Machine In India#Buy SORIN Stockert 3T at Best Price in India#Sorin 3t Heater Cooler#Sorin Stockert 3T Heater-Cooler#Sorin 3T Heater Cooler Systems#Stockert 3T Heater-Cooling System#About the Sorin Stockert 3T Heater-Cooler System#Sorin Stockert 3T | Heater/Cooler Systems#What is a Heater-Cooler Device?#Patient Cooling and Warming Systems#heater-cooler unit cardiac surgery#Patient Warming#Cooling in One System#Heater-cooler units used during cardiac surgery#Heater Cooler Device For Heart Lung Machine#Warming & Cooling Devices in Healthcare#Patient Cooling System#Patient cooling system - All medical device manufacturers#Heater Cooler Systems Supplier#Heater-Cooler Devices#heater-cooler device#sorin 3t heater-cooler service manual#heater-cooler perfusion#terumo heater cooler
0 notes
Text
Transformative Technologies: In Vitro Diagnostics in Focus
IVD refer to medical devices and tests that are used to analyze samples taken from the human body, such as blood, urine, and tissue. These samples are collected from patients and tested outside of a living body in controlled laboratory conditions. IVD assists in disease screening, diagnosis of infections like HIV, monitoring disease progression or regression, and making decisions regarding drug treatments and medical interventions.
Growing Demand and Market Size
The global IVD market was valued at $70 billion in 2020 and is projected to reach $126 billion by 2028, expanding at a CAGR of 7.3% during the forecast period. The rising burden of chronic and infectious diseases, technological advancements in miniaturization and automation, point-of-care testing, and personalized medicine are some of the key factors driving the growth of the IVD industry. Precision medicine and companion diagnostics are also creating new opportunities for IVD manufacturers to cater to unmet medical needs.
Emerging Technologies
Some of the emerging technologies revolutionizing the In Vitro Diagnostics landscape include:
Next-Generation Sequencing (NGS)
NGS allows the sequencing of millions of DNA fragments simultaneously at high speed and low cost. It is being widely used for genetic disease screening, cancer diagnosis through tumor mutational burden testing, infectious disease detection, pharmacogenomics, and non-invasive prenatal testing. Continuous advancements in NGS workflow automation, data analysis, and interpretation are making it more accessible for clinical use.
Lab-on-a-Chip Technology
Also known as microfluidics, lab-on-a-chip miniaturizes traditional benchtop laboratory tests onto a silicon chip a few square centimeters in size. It allows automation and parallel processing of multiple diagnostic assays with minimal sample volume requirements. Applications include point-of-care testing for infectious diseases and glucose monitoring. Further advancement can make lab-on-chip diagnostics affordable for use in resource-limited settings.
Digital and Molecular Diagnostics
The digitization of diagnostic processes allows automation and streamlining of pre-analytical, analytical, and post-analytical stages. Digital PCR, isothermal amplification techniques, and microarray-based molecular diagnostics offer high sensitivity and specificity for infectious disease detection, genetic disorders screening, and cancer monitoring. Integration of AI and machine learning is augmenting data analysis capabilities.
Advancement in Biosensors
Continued research into nanotechnology, materials science, and sensor fabrication is revolutionizing the development of biosensors for IVD applications. Electrochemical, optical, and mass-sensitive biosensors enable rapid, multiplexed, affordable, and on-site testing with high precision. Applications include glucose monitoring, genetic disease screening, cardiac marker testing, infectious agent detection for epidemics and bioterrorism threats.
Challenges and Standardization Needs
While emerging technologies hold immense potential to transform diagnostics, their clinical validation and regulatory approval remain long drawn processes. Achieving standardization in pre-analytical variables, performance metrics, quality control protocols, and data interpretation across decentralized locations poses difficulties. High initial investment and operational costs can delay the real-world adoption of advanced IVD technologies, especially in low to middle-income countries. Lack of skilled labor and infrastructure in resource-limited regions further hampers access to quality diagnostic services. Overcoming these challenges through partnerships, standardized guidelines, innovative business models, and human capital investments would be crucial to realize the full benefits of emerging IVD technologies.
Regulatory Changes and Global Harmonization
In vitro diagnostic regulators worldwide are aligning processes and requirements to facilitate the global development and distribution of new IVD technologies. The U.S. FDA is shifting from a risk-based to a total-product lifecycle approach through the implementation of the Verification and Validation framework. The European IVD Regulation establishes a single regulatory structure across EU markets. Global harmonization initiatives led by bodies like the World Health Organization aim to establish consistent standards and mutual recognition of approvals. Such regulatory changes intend to expedite patients' access to advanced diagnostics while maintaining pre-market evaluation of safety, efficacy, and performance.
Future Trends and Conclusion
The future of IVD looks promising with advancements spanning multiple omics technologies, digital platforms, lab miniaturization, and big data analytics. Integration of diagnostics into therapeutic strategies will become more prevalent. Radical new technologies like mobile health diagnostics, wearable biosensors, and molecular pathology could transform healthcare delivery models. Nonetheless, building robust research infrastructure, streamlining regulatory pathways, ensuring affordability, and addressing ethical issues would be pre-requisites to realize the full potential. IVD's crucial role in public health interventions and precision medicine will continue propelling innovations aimed at making diagnostics more accessible, non-invasive, rapid, accurate, and cost-effective.
0 notes
Text
North America Remote Cardiac Monitoring Market Analysis, Size, Share and Key Segments Poised for Strong Growth in the Future by 2030
Business Market Insights, “North America Remote Cardiac Monitoring Research Report | Forecast Year 2030” market research is now out for purchase. This report offers an exclusive evaluation of a range of business environment factors impacting market participants. The market information included in this report is assimilated and reliant on a few strategies, for example, PESTLE, Porter's Five, SWOT examination, and the effect of COVID-19 pandemic updates on the North America Remote Cardiac Monitoring.
North America Remote Cardiac Monitoring is evaluated based on current scenarios and future projections are added keeping forecast duration in consideration. This report integrates the valuation of North America Remote Cardiac Monitoring size for esteem (million USD) and volume (K Units). Research analysts have used top-down, bottom-up, primary, and secondary research approaches to evaluate and approve the market conclusions.
Detailed scrutiny of market shares, optional sources, and basic essential sources has been done to integrate only valid facts. Central participants contending in the global North America Remote Cardiac Monitoring are
This research further reveals strategies to help companies grow in the North America Remote Cardiac Monitoring.
Key objectives of this research are:
To present market dynamics including drivers, challenges, threats, and opportunities in the North America Remote Cardiac Monitoring.
To analyze the sum and estimation of the worldwide North America Remote Cardiac Monitoring
Based on key aspects, market segments are added.
The competitive analysis covers key players and their strategies.
To examine the Global North America Remote Cardiac Monitoring for business potential and strategic outlook.
To review the Global North America Remote Cardiac Monitoring size, key market regions, end-users, and statistical details.
To offer strategic recommendations based on the latest developments, and trends in the North America Remote Cardiac Monitoring.
Briefing Note on COVID-19 Analysis
Our latest perspectives on the Covid-19 pandemic and its impact across industries are analyzed in a dedicated chapter. The insights in this chapter intend to help the organization to prepare for the next normal. The COVID-19 endemic has turned the business focus to sustainable and inclusive growth. We present a thorough analysis of the pandemic downstages followed by recovery strategies to accelerate prosperity. Companies need to enhance their efforts in the ### by rewarding the change.
What all adds up to the credibility of this research?
Comprehensive summary of present North America Remote Cardiac Monitoring condition.
Accurate estimations on market revenue forecasts and CAGR to rationalize resources.
Regional reporting to uncover new markets for business
Competition analysis aims to help corporations in a modest edge.
Facts-based crystal-clear insights for business success.
The research is modified as per business necessities.
Access to PDF, and PPT formats of this research.
For any research and consultation queries reach out to us at [email protected]
To Summarize the Key Highlights of this Report:
Market Insights-Market Size & Forecast by Revenue | Forecast Year
Market Dynamics – Foremost trends, market drivers, challenges, and opportunities
Market Segmentation –By product, types, end-user, applications, segments, and regions
Competitive Landscape – Top players and other key vendors
Briefing on the Impact of Covid-19
Recent Developments
Strategic Recommendation
About Us:
Business Market Insights is a market research platform that provides subscription service for industry and company reports. Our research team has extensive professional expertise in domains such as Electronics & Semiconductor; Aerospace & Defense; Automotive & Transportation; Energy & Power; Healthcare; Manufacturing & Construction; Food & Beverages; Chemicals & Materials; and Technology, Media, & Telecommunications
0 notes
Text
Comprehensive First Aid Training & AED Defibrillator in Ontario, Canada
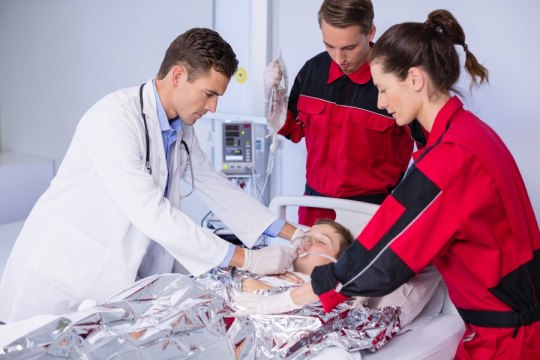
Defibrillators, or AEDs, are portable machines that give electric shocks to help restart normal heart rhythm when someone is in cardiac arrest. They’re safe for anyone to operate and give verbal instructions to guide you. AED Defibrillator in Ontario have made saving lives during heart attacks possible even before paramedics arrive.
Understand AED Basics
An AED administers controlled electric shocks through adhesive electrode pads placed on the chest to help stabilize irregular heartbeats until emergency medical teams arrive. The devices provide simple audio direction and screen prompts guiding users through operation.
Once powered on, AEDs first analyze heart rhythm through the pads to determine if an abnormal pattern exists requiring intervention. The device will only deliver a shock as appropriate.
AED models contain slight differences in features like:
Fully or semi-automated functionality
Child/infant electrode pad compatibility
Data recording and transmission capacities
Battery lifespan
But core functions follow similar user-friendly protocols applicable across machines.
AED Training and sales in Canada
National and provincial health agencies regulate availability, maintenance, signage, and oversight protocols of AED Defibrillator in Ontario. Legislation aims to standardize public access from malls and airports to schools and workplaces for timely emergency use.
Any organization or building with high traffic must provide on-site AED training and sales. Facility managers should understand provincial requirements on registering devices, maintaining functionality per manufacturer guidelines, notifying emergency services, and posting visible signage.
Selecting Reliable AED Suppliers
Various vendors across Canada offer AED sales both online and through community showrooms. Prices range widely from $1,200 to over $2,000 depending on features. Buyers should assess suppliers on:
Device approvals - Confirm Health Canada, FDA or European Union compliance certifications.
Equipment guarantees - Multi-year warranties on hardware and software reflect reliability.
Service longevity - Well-established vendors better support long-term maintenance needs.
Customer support – Check availability of contact channels like live chat, email ticketing, phone assistance.
Price transparency – Review quotes clearly detailing equipment costs, maintenance plans, extras like wall mounting.
Ideally suppliers also possess manufacturer partnerships enabling direct equipment purchases without middleman markups. This ensures the lowest pricing alongside full factory warranty coverage.
The Importance of AED and First Aid Training
Once organizations acquire AED units, comprehensive training ensures employee confidence and quick response during emergencies. Proper instruction covers:
Recognizing signs of cardiac arrest
Operating AED devices safely
Following voice/text command prompts
Applying electrode pads correctly
Understanding basic maintenance like battery and pad replacement
Safely delivering CPR and clearing airways
Monitoring patients until emergency responders arrive
Certified first aid training centers offer in-person group classes or web-based self-paced courses for convenience. Custom modules allow targeting instruction to adults, children or infants as needed. Dedicated shock training prepares people for emotionally stressful situations when activating the AED process.
Staying Current with All First Aid Training in Canada
While basic AED and first aid certification lasts two to three years, regular refreshers ensure personnel maintain lifesaving skill levels. People forget nearly 80% of new information within one month without review or practice.
Refreshers courses are key every year or two because skills get rusty fast without practice. So even after initial certification, regular re-training helps personnel react instinctively in an emergency. For national companies, e-learning makes re-training efficient across many locations.
By investing in good AED systems and ongoing all first aid training in Canada, Canadian organizations show safety is a priority. Being prepared to respond quickly during heart attacks can truly save lives.
0 notes
Text
Why IV Cannulas are Essential in Modern Healthcare

In modern healthcare, IV cannulas play a crucial role in delivering vital medications and fluids directly into the bloodstream. Their importance cannot be overstated, as they provide a quick and efficient way to administer treatment to patients in need. In this blog post, we will explore benefits of IV cannulas and why they are essential in medical care today.
What are the IV cannulas?
First things first, let’s talk about what IV cannula actually are. An IV cannula, short for intravenous cannula, is a small, flexible tube used to administer medications and fluids directly into a patient’s bloodstream. This important medical device is inserted into a vein to provide quick and efficient access for delivering essential treatments. Understanding the basics of IV cannula is crucial for healthcare professionals to ensure proper patient care and treatment outcomes.
The Importance of IV Cannulas in Modern Healthcare
IV cannula represent a cornerstone of modern healthcare delivery, embodying the intersection of innovation, efficacy, and patient-centered care. Their versatility, reliability, and safety make them indispensable tools for healthcare providers across diverse clinical specialties, facilitating optimal patient outcomes and fostering a culture of excellence in healthcare delivery. As medical technology continues to advance, IV cannulas will undoubtedly remain essential components of the therapeutic armamentarium, shaping the future of patient care for generations to come.
Benefits of IV Cannulas in Patient Care
One of the biggest benefits of IV cannula is their versatility. They can be used for a wide range of treatments, including hydration, medication administration, blood transfusions, chemotherapy and more. They also allow for continuous monitoring of a patient’s condition, making it easier for healthcare providers to adjust their treatment plan as needed. Explore our high-quality IV cannula – crafted by industry-leading manufacturers at KDL.
Advancements in IV Cannula Technology
Thanks to advancements in medical technology, Intravenous needle have come a long way in recent years. New materials and designs have made them more comfortable for patients and easier for healthcare providers to insert. This means less pain and fewer complications for everyone involved.
Read More : The Benefits of Using Huber Needles for Long-Term IV Therapy
The Role of IV Cannulas in Preventing Infections
Infection control is a top priority in healthcare settings, and it play a key role in preventing the spread of harmful bacteria. By using sterile techniques and regularly changing IV sites, healthcare providers can reduce the risk of infections and keep patients safe during treatment.
IV Cannulas in Emergency Situations
When every second counts, it can be a lifesaver. In emergency situations like trauma or cardiac arrest, quick access to a patient’s bloodstream can mean the difference between life and death. It allows for rapid administration of critical medications and fluids, giving patients the best chance at recovery.
Conclusion: The Future of IV Cannulas in Healthcare
In conclusion, the future of IV cannulas in healthcare looks promising with advancements in technology and materials. Healthcare providers can expect improved patient comfort, reduced infection rates, and increased efficiency in delivering intravenous therapy. As the healthcare industry continues to evolve, it plays a crucial role in enhancing patient care and outcomes. Stay informed and embrace the latest innovations to ensure optimal use of IV cannulas in the ever-changing landscape of healthcare.
Source : Why IV Cannulas are Essential in Modern Healthcare
#IV Cannulas#Healthcare#medical technology#Healthcare Essentials#Medical Devices#Medication#iv cannula needle#intravenous cannula#iv cannula manufacturers
0 notes
Text
Navigating the Landscape of ICU Monitors: Factors to Consider When Evaluating Price
In critical care settings, the Intensive Care Unit (ICU) monitor is a vital tool that provides continuous monitoring of patients' vital signs and physiological parameters. From cardiac rhythm to oxygen saturation levels, ICU monitors play a crucial role in ensuring timely intervention and optimal patient care. As healthcare facilities strive to equip their ICUs with state-of-the-art monitoring systems, understanding the factors that influence ICU monitor prices is essential. Let's explore the key considerations when evaluating the price of ICU monitors.
1. Technology and Features:
ICU monitors vary in terms of technology and features, ranging from basic vital signs monitoring to advanced multi-parameter monitoring systems. The price of an ICU monitor is often reflective of its technological sophistication and the breadth of features it offers. Factors such as touchscreen interfaces, wireless connectivity, customizable alarm settings, and integration with electronic medical record (EMR) systems can contribute to higher price points.
2. Brand and Reputation:
Established brands with a reputation for quality and reliability often command higher icu monitor prices. These brands invest in research and development to continuously innovate and improve their monitoring solutions, which may justify the premium pricing. Additionally, renowned brands may offer better warranty and support services, adding further value to their products.
3. Durability and Longevity:
The durability and longevity of an icu monitor prices are critical factors to consider when evaluating its price. High-quality monitors built with robust materials and components may have a higher upfront cost but can offer long-term reliability and reduced maintenance expenses. Investing in a durable ICU monitor can result in cost savings over the product's lifecycle.
4. Scalability and Customization:
Healthcare facilities may have unique requirements for ICU monitoring based on their patient population, specialty areas, and workflow preferences. ICU monitors that offer scalability and customization options, such as modular expansion capabilities and configurable display layouts, may come at a premium price. However, the ability to tailor the monitoring system to specific clinical needs can enhance efficiency and clinical outcomes.
5. Regulatory Compliance:
Compliance with regulatory standards and certifications, such as FDA approval and CE marking, is essential for ICU monitors to ensure patient safety and regulatory compliance. Monitors that meet stringent regulatory requirements may undergo rigorous testing and certification processes, which can contribute to higher manufacturing costs and, subsequently, higher icu monitor prices.
Conclusion:
In conclusion, the price of ICU monitors is influenced by various factors, including technology and features, brand reputation, durability, scalability, and regulatory compliance. Healthcare facilities must carefully evaluate these factors in conjunction with their budgetary considerations and clinical requirements when selecting an ICU monitoring solution. By understanding the key considerations that drive ICU monitor prices, healthcare professionals can make informed decisions to ensure optimal patient care and clinical outcomes in critical care settings. Explore the range of ICU monitors available in the market, weigh the cost-benefit considerations, and invest in a monitoring solution that aligns with your facility's needs and priorities.
0 notes
Text
Cardiac Monitor Manufacturers
A comprehensive range of cardiac monitors, as well as a variety of related products and services on the Hospital Product Directory platform. To help you refine the results for cardiac monitor manufacturers, Cardiac Monitor suppliers, and Cardiac Monitor dealers in India, Visit us for more details. Hospital Product Directory is a business directory featuring listing Medical Equipment's Indian manufacturers and suppliers of India with their product profiles and contact details.
0 notes
Text
Saving Lives: The Role of Automated External Defibrillator (AED) Machines in Cardiac Emergencies
Introduction: In the realm of emergency medical response, Automated External Defibrillator (AED) machines stand as powerful devices capable of swiftly restoring normal heart rhythm in cases of sudden cardiac arrest (SCA). This article delves into the significance of AED machines, their operation, and their profound impact on improving survival outcomes during cardiac emergencies.
Understanding AED Machines: AED machines are portable electronic devices designed to detect abnormal heart rhythms and deliver a therapeutic shock to the heart to restore its normal rhythm. They are equipped with adhesive electrode pads that are placed on the chest of the individual experiencing cardiac arrest.
Functionality and Operation:
Upon activation, AED machines guide users through the rescue process with clear voice prompts and visual cues.
The device analyzes the heart rhythm and determines whether a shock is necessary.
If a shock is advised, the machine delivers a controlled electric shock through the electrode pads to restore normal cardiac function.
Importance in Cardiac Emergencies:
Prompt deployment of AED machines is critical in cases of sudden cardiac arrest, as every minute without defibrillation decreases the chance of survival by approximately 10%.
AED machines can be utilized by trained individuals and bystanders with minimal training, making them accessible in various settings, including public spaces, workplaces, and healthcare facilities.
Impact on Survival Rates:
Studies have shown that early defibrillation with an AED within the first few minutes of cardiac arrest can increase survival rates by up to 70%.
By enabling immediate intervention before professional medical help arrives, AED machines play a vital role in the chain of survival for individuals experiencing cardiac emergencies.
Maintenance and Accessibility:
Regular maintenance and periodic checks are essential to ensure the readiness and functionality of AED machines.
This includes monitoring battery status, electrode pad expiration dates, and conducting routine self-tests as recommended by the manufacturer.
Conclusion: Automated External Defibrillator (AED) machines serve as indispensable tools in cardiac emergencies, offering a lifeline to individuals experiencing sudden cardiac arrest. With their user-friendly operation, rapid deployment, and proven efficacy in increasing survival rates, AED machines empower communities and healthcare facilities to respond effectively to critical cardiac events. Explore high-quality AED machine options available at HospitalStore to equip your environment with the tools needed to save lives in cardiac emergencies.
0 notes
Text
Beyond Imaging: The Therapeutic Potential of Radiopharmaceuticals in Cancer Treatment

Radiopharmaceuticals: Enabling Diagnosis and Treatment through Nuclear Medicine
Nuclear medicine is a medical specialty that utilizes tiny amounts of radioactive material, known as radiopharmaceuticals, to diagnose and treat diseases. These radioactive compounds, when administered to the patient, allow physicians to non-invasively visualize organs, bones, or tissues within the body. Radiopharmaceuticals play a critical role in enabling the promising field of nuclear medicine to deliver accurate diagnostic information and targeted therapies to patients.
What are Radiopharmaceuticals?
Radiopharmaceuticals are radioactive compounds consisting of a radioisotope bound to or incorporated into a molecular structure known as a ligand or carrier. The radioisotope, usually in the form of a radionuclide such as technetium-99m, emits radiation as its unstable nucleus decays. The ligand delivers the radioisotope to specific organs, tissues, or cellular targets within the body. The two main types of radiopharmaceuticals are diagnostic and therapeutic.
Diagnostic Radiopharmaceuticals in Nuclear Medicine Imaging
Nuclear medicine imaging plays a vital role in disease diagnosis through the use of radioactive tracer molecules. The most widely used diagnostic radiopharmaceutical is technetium-99m (Tc-99m), which emits gamma rays that can be tracked by a gamma camera. Tc-99m has near-ideal radiation properties and is easily produced by many nuclear pharmacies onsite at hospitals using generators. It is incorporated into ligands that target specific organs and tissues. Some common examples include Tc-99m sestamibi, which localizes to heart muscle cells and is utilized in cardiac imaging, and Tc-99m sulfur colloid, which localizes to liver and spleen allowing imaging of these abdomional organs.
Therapeutic Radiopharmaceuticals for Cancer Treatment
Radioactive atoms have also found applications in directly treating certain types of cancer. Radionuclide therapy harnesses the cell-killing effects of radiation exposure. Common radiopharmaceutical therapies include iodine-131 to treat thyroid cancer and certain types of non-Hodgkin's lymphoma, and radium-223 for bone metastases from castration-resistant prostate cancer.
Ensuring Safety in Radiopharmaceutical Production and Delivery
Strict guidelines must be followed to guarantee the safe production, quality control, transportation, clinical use, and disposal of radioactive drugs. All radiopharmaceutical production facilities and nuclear pharmacies are regulated by bodies like the International Atomic Energy Agency and the United States Nuclear Regulatory Commission. Healthcare workers who handle radiopharmaceuticals are specifically trained and monitored using personal dosimetry badges to ensure radiation exposure levels remain very low.
The future of Radiopharmaceuticals
Looking ahead, new radioisotopes and radiolabeling techniques continue expanding the toolbox of nuclear medicine. Targeted alpha therapies hold promise in oncology, and radiopharmaceuticals image and treat neurodegenerative diseases. Cellular and molecular radiotracers could enable earlier disease detection. Combination therapy using radiolabeled drugs with immunotherapy or chemotherapy may offer enhanced treatment responses. Advances in manufacturing technology are improving radiopharmaceutical production capabilities and availability worldwide.
In conclusion, radiopharmaceuticals are instrumental diagnostic and therapeutic tools that underpin the rapidly advancing field of nuclear medicine. Strict safety guidelines ensure their reliable clinical use. Continued innovation promises to further expand radiopharmaceutical applications to benefit many more patients worldwide in the future
0 notes
Text
What Is ICD ? Who Needs It?
An Implantable Cardioverter Defibrillator (ICD) is a small electronic device that is placed inside the body, usually in the chest area, to continuously monitor and regulate the heart’s rhythm. It is primarily used to treat life-threatening heart rhythm disorders called ventricular arrhythmias, which can cause the heart to beat too fast (ventricular tachycardia) or irregularly (ventricular fibrillation).
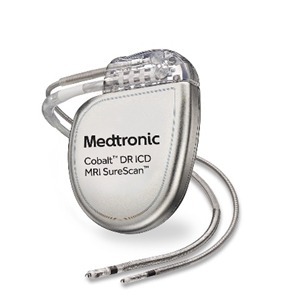
The ICD consists of a pulse generator and one or more leads. The pulse generator contains a battery and circuitry that constantly monitors the heart’s electrical activity. The leads are thin insulated wires that are threaded through blood vessels into the heart and are responsible for sensing the heart’s electrical signals and delivering electrical shocks or pacing pulses when necessary.
The ICD operates in two main modes: monitoring and therapy. In the monitoring mode, it continuously monitors the heart’s rhythm and records any abnormal electrical activity. If it detects a potentially life-threatening arrhythmia, it can deliver a low-energy electrical shock, known as defibrillation, to restore a normal rhythm. The shock is delivered through the leads and is intended to stop the dangerous arrhythmia and allow the heart to resume a regular rhythm.
In addition to defibrillation, the ICD can also function as a pacemaker, delivering low-energy pacing pulses to regulate the heart rate if it detects a slow or irregular rhythm that does not require defibrillation. This feature helps ensure that the heart maintains a steady and adequate blood flow.
ICDs are typically recommended for individuals who have survived cardiac arrest, have experienced certain types of dangerous arrhythmias, or are at high risk of sudden cardiac death due to underlying heart conditions. They are implanted by a minor surgical procedure, usually under local anaesthesia. Once implanted, the ICD is programmed by a healthcare professional to suit the individual’s specific needs and can be monitored and adjusted during regular check-up appointments.
It’s crucial to note that the information shared is a general overview; details about the ICD and its indications may vary based on the manufacturer, technological advancements, and individual patient considerations. If you or someone you know needs an ICD, consulting with a Best Cardiologist in Pune ensures accurate, personalized information aligned with the latest medical guidelines and practices.
#cardiologist in pune#interventional cardiologist#cardiologist#Dr. Rahul Sawant#best cardiologist in pune#pacemaker
0 notes
Text
What is the classification process used by CDSCO (Central Drugs Standard Control Organization) for medical devices?
Medical devices in India undergo classification by the Central Drugs Standard Control Organization (CDSCO), based on criteria such as intended use and potential risks. The classifications help establish regulatory requirements, and the main categories are outlined as follows:
Class A:
Represents low-risk devices, including items like thermometers and tongue depressors.
Class B:
Encompasses low to moderate-risk devices, such as blood pressure monitors and syringes.
Class C:
Signifies devices with moderate to high risk, including surgical drapes and bone fixation plates.
Class D:
Indicates high-risk devices, including cardiac stents and heart valves.
Each class comes with its specific regulatory requirements, playing a crucial role in determining the regulatory pathway for obtaining CDSCO approval or registration. Manufacturers need to understand their medical device's classification to ensure compliance with applicable regulations, referencing the latest CDSCO guidelines for accurate and current information.
0 notes