Link
Heart Health Intelligence scored $2.2 million in seed funding to develop its connected toilet seat aimed at collecting cardiac data points.
This new infusion of cash was led by Bemis Manufacturing Company, with participation from LaunchNY, RIT Venture Fund, Tech Coast Angels, Excell Partners and Impact Capital of New York. Bemis Manufacturing will be teaming up with the startup to produce the seats.
WHAT THEY DO
The Rochester, New York-based startup is focused on developing the Heart Seat, which combines a toilet seat with a cardiovascular monitoring system. Patients can use the tech and have their heart rate, blood pressure, cardiac output, ECG and blood oxygenation checked.
The tool is designed to go into homes, but the data can be used in various ways. The data can be sent to families, caregivers and healthcare providers – or to patients with heart failure. Additionally, the seat can detect deterioration and send out alerts.
Currently the tool is still under development. However, last year the company teamed up with the University of Rochester Medical to test the tech. The results from this study were published in JMIR and found that, over an eight-week period, the system demonstrated clinical-grade accuracy for measurements of blood pressure, stroke volume and blood oxygenation when compared with their respective gold standards.
WHAT IS IT FOR?
The new funds will be used to help the technology go through the process of FDA clearance, then get it up and running for commercialization.
MARKET SNAPSHOT
Individuals are increasingly beginning to monitor heart health outside the home. One of the most popular ways to do this is through wearable technology. In 2018, Apple landed FDA clearance for both an atrial fibrillation-detecting algorithm and an ECG that will be built into the company's Series 4 Apple Watch.
In terms of clinical-grade technology, just last week Swiss startup Aktiia scored $6.1 million to bring its continuous-blood-pressure-monitoring bracelet to market.
ON THE RECORD
“We are going to make a tangible difference in the lives of patients and healthcare providers,” Nicholas Conn, Heart Health Intelligence CEO, said in a statement. “The strong partnerships we are forming now continue to add to our momentum and I’m incredibly excited for all the good that our technology has the potential do.”
#Digital Health#Health Innovation#Heart Health Intelligence#Health Tech Startups#Connected Toilet Seat#IoT
1 note
·
View note
Link
Pear Therapeutics announced this morning that Somryst, its prescription digital therapeutic for adults with chronic insomnia, has received marketing authorization from the FDA.
The treatment provides cognitive behavioral therapy for insomnia (CBTi) alongside personalized, algorithm-generated sleep restriction recommendations. The digital therapeutic was reviewed through the agency's 510(k) pathway and, notably, is also the first product to pass through the FDA's experimental Software Precertification Pilot Program.
Somryst's tailored CBTi is delivered over the course of nine weeks through a smartphone, tablet or similar device. It is intended to treat the symptoms of patients aged 22 years or older with chronic insomnia, and is only available by prescription from a licensed healthcare provider.
Pear's regulatory submission was accompanied by two randomized controlled trials of the digital therapeutic, the company said in its announcement. The first, published in JAMA Psychiatry, measured six- and 12-month insomnia symptoms among 303 patients assigned the treatment or an active control. The second, published in Lancet Psychiatry, involved 1,149 patients, and demonstrated benefits over controls persisting as long as 12 months among those assigned the 9-week treatment.
While the company doesn't yet have a time line for when the product will hit the market, Dr. Yuri Maricich, Chief Medical Officer at Pear, said that Pear's goal is to follow a commercialization and distribution model roughly similar to those of its other two products, reSET and reSET-O. In this approach, a provider would send the prescription to Pear's "Patient Service Center," which staffs specialists who guide the patient through downloading the app through the App Store or Google Play Store, entering their prescription access code and then using the app-based therapy itself.
WHY IT MATTERS
CBTi is a listed as a first-line treatment for chronic insomnia in clinical guidelines from the American College of Physicians and the American Academy of Sleep Medicine alike. The downside, Maricich said, is that the demand for these behavioral health treatments greatly outstrip the 500 clinicians certified to provide CBTi in the U.S.
"The majority of patients aren't able to get treatment," he said. "Even if you are lucky enough to get [it], we often hear both from the clinicians, as well as patients, that they have a waiting list that oftentimes stretches beyond a year. So there's a really huge need."
As a digital treatment, Somryst can be easily scaled and delivered to patients who otherwise might have been prescribed a more traditional drug for their medication. With this in mind, he said that the company will be focusing on raising awareness of the product among a broad range of providers, and primary care doctors in particular.
"Primary care clinicians in general actually treat the bulk of chronic insomnia by volume, but typically because they haven't had good treatment options ... they'll just say, 'Well, why don't you take this pill for a little bit?' because they just don't have access," Maricich said. "I think the main point here is that there is going to be a broad group of clinicians who will now have something they can actually provide, that they know is the recommended [treatment]. That will just be part of [Pear's] overall approach: How do we make sure clinicians across specialty types know about it and have access?"
For Pear, Maricich said that Somryst clearance represents a major portfolio expansion beyond addiction treatment and into the broader neurobehavioral intervention space. For digital health at large, the digital therapeutic is now the first and only product put through the ringer of the Precertification Program's 1.0 Working Model.
Maricich spoke at length about what the Pre-Cert process was like for Pear and what others might expect when the pathway is opened to the public (check back with MobiHealthNews tomorrow for a full Q&A write-up). While the prescription digital therapeutics company's experience with the early Working Model and a simultaneous 510(k) was certainly atypical, he did note that the program's "Excellence Assessments" will likely be more expansive than some might be expecting.
"If a company was getting ready to go to an Excellence Appraisal and thought, 'Oh, we just need our quality people to show up, this is just an overview of our quality systems,' it was much larger than that," he said. "It was, writ large, what does high-quality software that impacts health look like? And, how do we think about that, not just from a quality perspective, but from a clinical perspective, from an organizational level around monitoring and refining our actual development process in an agile way to make the software better over time."
THE LARGER TREND
Pear announced that Somryst would be putting the FDA's experimental framework through its paces back in July, and was tapped to participate in the pilot as far back as 2017. The program itself was scheduled to conclude its one-year trial of the Working Model with the new decade, although the last update from the agency came with its six-month check-in during the summer.
Pear itself went through a bit of a shakeup last fall when its digital-therapeutics commercialization arrangement with Novartis' Sandoz came to an abrupt end. However, the company has been steadfast in its ability to commercialize and distribute its product without the pharma's support, and even announced a flurry of tech acquisitions around the start of the new year.
ON THE RECORD
“The FDA’s authorization of our third PDT, Somryst, demonstrates our ongoing commitment to redefine disease treatment through the development of PDTs,” Dr. Corey McCann, president and CEO of Pear Therapeutics, said in a statement. “Now, more than ever, easily-accessible treatment options are imperative for patients suffering from chronic conditions. Pear has built the capabilities, pipeline, and platform to allow us to build PDTs that help patients across a variety of disease areas.”
#Digital Therapeutics#DTx#PDT#Pear Therapeutics#Somryst#MS#Multiple Sclerosis#FDA Pre-Certification#Health Innovation#Digital Pharma
0 notes
Video
youtube
Convening innovative public and private leaders working to address the COVID-19 pandemic.
11:00AM -11:10AM: Opening Remarks
11:10AM -11:35AM: What Are Governments, NGOs, and the Private Sector Doing To Address The Pandemic? What Do We Need To Know?
11:35AM - 11:45AM: The Front Door: How Do We Find Who Is Sick?
11:45AM-12:10PM: What Should We Do To Care For People With COVID-19?
12:10PM-12:20PM: Supporting Care Infrastructure: How Do We Coordinate In A Pandemic?
12:20PM-12:45PM: How Do We Help People In A Community Experiencing Economic Turmoil and Medical Uncertainty?
12:45PM-12:55PM: Virtual Life: How Do We Continue to Live, Receive Care, and Explore When We're Trapped At Home?
12:55PM - 1:05PM: How is Data Science and Technology Being Leveraged to Help Businesses and Consumers Make Smart Decisions in a Time of Financial Uncertainty?
1:05PM-1:20PM: Use Case - OSF HealthCare & CareSignal
1:20PM-1:30PM: Closing Remarks
Featured Speakers
Alan Gilbert - Vice President of New Business Initiatives & Drivers of Health Strategy at Anthem
Alexandra Drane - Co-founder and CEO of Rebel Health and ARCHANGELS
Blake Marggraff - CEO at CareSignal (formerly known as Epharmix)
Brian Anderson - Chief Digital Health Officer at MITRE Corporation
Charlotte Yeh - Chief Medical Officer at AARP
Jennifer Joe - Physician and CEO of Vanguard Health
John Brownstein - Chief Innovation Officer at Boston Children's Hospital
Juliette Kayyem - Co-founder and CEO of Grip Mobility; National Security Analyst at CNN
Margaret Bourdeaux - Research Director at Harvard Medical School
Meg Barron - Vice President of Digital Health Strategy at American Medical Association
Rob Jennetten - Director of Innovation Partnerships at OSF Healthcare
Vanessa Kerry - Founder and CEO of Seed Global Health
Featured Innovators
Allison Martin - CEO of UDoTest
Andrew Le - CEO of Buoy Health
Doug Williams - Chief Product Officer of 1upHealth
Eran Orr - CEO of XRHealth
Gideon Taub - CEO of Pinkaloo
Grace Andruszkiewicz - Director of Marketing and Partnerships at Rendever
Khristina Butenko - Head of Product and Business Strategy at 1upHealth
Kristi Ebong - SVP of Corporate Strategy at Orbita
Laura Kornhauser - CEO and Co-Founder of Stratyfy
Michael Docktor - Founder & CEO of Dock Health
Sam Tackeff - COO of Ompractice
Seth Brecher - Head of Partnerships and Customer Success at Edmit
Tanya Perkins - COO of Tembo Health
0 notes
Video
youtube
Purchase e-book here: https://leanpub.com/thefutureofpharma
An e-book that explains how disruptive technologies and emerging trends such as robotics, artificial intelligence, 3D printing, precision medicine or patient design will impact the manufacturing and distribution of pharmaceuticals in order to prepare successfully for a better future of healthcare.
We designed this e-book to serve as a collection of relevant examples, best practices and exciting ideas that can help any pharmaceutical company prepare for change. Many pharma companies have been trying to hop on the “digital train”. This e-book was meant to prove that instead of a train of innovation, stakeholders should think in terms of spaceships and while there is still time to embrace digital health and patient empowerment, those that do it faster will get exponentially ahead of their competitors.
0 notes
Link
Volunteers from Amazon, Alphabet, Apple and other tech companies worked every night for a week to make a website called covidnearyou.
The team collaborated closely with epidemiologists, including John Brownstein from Boston Children’s Hospital.
covidnearyou.org asks healthy and sick people to share their symptoms.
In the last week, a group of thirty volunteers from tech companies like Apple, Amazon and Alphabet put together a website called “covidnearyou” that aims to track the coronavirus as it spreads.
The idea started when Prem Ramaswami, the head of product at Alphabet’s Sidewalk Labs, and his wife, started feeling sick more than a week ago. When he tried to get a test for the coronavirus, his doctor told him that would not be possible. According to Ramaswami, he was denied access to the test because he hadn’t been in touch with anyone who had tested positive.
Ramaswami, who previously worked on health projects at Google, wondered how he could help others in the same boat. So he got in touch with John Brownstein, an epidemiologist and chief innovation officer at Boston Children’s Hospital, to volunteer his services. Brownstein is well-known to the tech world as he has consulted with companies like Google and Uber on public health projects for years, including the Google Flu Project, which tracked the spread of the flu.
Once they jumped on a call, Brownstein informed him about a website that was already underway to monitor influenza, called “flunearyou.” The pair decided to co-opt the underlying technology for better COVID-19 monitoring, given the current gap in testing. That led to the idea of developing “covidnearyou.”
But Ramaswami realized he couldn’t do it on his own. So he pulled together a group of friends and acquaintances from the tech world to help out. Employees from Apple, Amazon, MongoDB, CloudFlare, Alphabet and other tech companies agreed to build the site, but defer to the public health experts on the content.
“I’m a tech guy, not a doctor,” said Ramaswami, who is more focused on civic-tech these days at Sidewalk Labs. “We are here to help the medical experts and take their direction.”
After a week of work, the team, which includes designers, engineers and marketers, are now ready to go live with covidnearyou.org. The site is recruiting people from across the U.S. to share whether they’re experiencing symptoms or not, as well as some demographic information.
If they can get enough people to use the site – and the goal is to reach 100,000 users – they hope to fill in some of the gaps in reporting due to the lack of testing.
“Without widespread testing, we don’t have a clear picture of where the illness is, ” Brownstein explained. “We are basically flying blind.”
Healthy individuals who participate are asked to fill out information about their gender, age and zipcode, and whether they’ve received a flu vaccine. The site also includes a map of the various states and shows where the outbreaks are.
Those who are feeling sick are prompted to describe their symptoms, such as fever, a cough, or shortness of breath. They are also asked if they’ve traveled recently, and if they’ve been in direct contact with anyone diagnosed with COVID-19.
In future iterations, the team plans to add more symptoms as medical professionals release more information about the coronavirus. For instance, some doctors are now saying such as digestive ailments and lack of smell should be added the list that the public should know about, alongside fever and respiratory issues.
According to Brownstein, the data will be shared with public health groups and not with the various tech companies.
As of this morning, about 10,000 people had provided their health status to covidnearyou, said Brownstein, and the majority of them are healthy. If usage keeps growing, he believes that the data-set will be large enough to be useful to public health officials.
The project’s volunteers say they are continuing to work on the site while juggling their day jobs.
“Many of us are working on this from 3pm to 9pm after work, and our spouses are helping take on the load with childcare,” said Ramaswami. “But people in tech are hungry to help out right now.”
#Digital Innovaiton#Health Innovation#ComingTogetherForCOVID#COVID-19#Amazon#Apple#Alphabet#Boston Childrens Hospital#covidnearyou
0 notes
Link
Evidation Health, a technology company that generates insights from real-world behavioral data collected through devices, has kicked off a new effort to better understand individuals' health behaviors and concerns throughout the COVID-19 pandemic.
Yesterday the company announced that it has recruited 100,258 people living in the U.S. into a longitudinal survey through the Achievement app, Evidation's patient engagement and rewards product for health organization customers.
These participants – a subsample of the app's nearly four million users who live in all 50 states and the District of Columbia – will be regularly providing their COVID-19 perceptions, changes in their behavior and any device-tracked activity data that is synced with the app.
“One of the great things about Achievement is our ability to layer permissioned data from connected devices and apps with self-reported outcomes we capture through surveys," Ernesto Ramirez, senior data scientist at Evidation Health, told MobiHealthNews in an email. "The trust and transparency we build with our members allows us to conduct novel research into the relationship between person-generated health data and important health conditions and outcomes like influenza and COVID-19.
The digital survey has already collected some baseline data in its first few days, the company wrote on its website. For instance, of respondents answering between March 12 and March 15 fewer (23%) said they believed that the U.S. was prepared or very prepared for the outbreak (23%), while roughly half ( said they believed that the virus represented a major threat to public health. Respondents said that they were generally washing their hands more frequently during the previous seven days (64%), but less often reported that they were avoiding large gatherings (34%).
There were also differences in replies between participants who did or did not have health insurance. The latter group more often said that they would not seek care if they developed coronavirus symptoms (8% versus 21%), and those who would more often said that they would most likely go to an emergency room (19% versus 26%).
A state-by-state breakdown of participant responses also illustrated differences in opinion among the populace. Here, Evidation highlighted the contrast between Washington, D.C. and Louisiana, which as of March 15 had the third- and fourth-highest number of cases per 100,000 residents. The former's respondents were the least assured in the country's preparedness for the disease and most often reported preventive behavior changes, while the latter's residents were among the most confident, and did not change their behaviors to a similar extent.
WHY IT MATTERS
The COVID-19 pandemic is escalating at a rapid pace, with the CDC disclosing thousands of new cases reported within the last day alone. Evidation's approach to polling can quickly yield a large quantity of information from generally decentralized responders, and complement those qualitative responses with quantitative activity data collected by their devices.
"Our ability to tap into a large virtual cohort of Americans shows the potential for leveraging new models of engagement and data collection not only for research purposes but meaningful and rapidly scalable public health surveillance," Ramirez said. "And because we're able to communicate with our members, we can track how behaviors and attitudes change over time as social and health dynamics shift in the future.”
With that being said, the strengths of these types of large, app-based studies can come at a cost. According to a recent meta-analysis of eight digital studies with similar designs, these approaches are often subject to low retention rates and participant samples that often skew younger and whiter than the general population.
THE LARGER TREND
Even in the earlier days of COVID-19's U.S. spread, health organizations and startups alike had developed a number of data-driven tools to track the outbreak. In February, Boston Children's Hospital's John Brownstein and Buoy Health's CEO Dr. Andrew Le described one such example that combines the former's data science tools with the latter's triaging chatbot.
For its own part, Evidation Health has a hefty collection of app-based health studies both logged and ongoing. Some of these have also been conducted in collaboration with big names. It was just a few weeks ago that its name was attached to Apple and Johnson & Johnson's Heartline Study of Medicare seniors.
0 notes
Link
Drugmaker Bristol-Myers Squibb has become the latest large drugmaker to team up with a maker of digital therapeutics, with an aim to create programs for cancer patients.
Cambridge, Massachusetts-based Voluntis said Tuesday that it would work with New York-based BMS to use its Theraxium Oncology system to create and investigate the programs, centered on a mobile app for patients, with the goal of supporting management of symptoms and remote monitoring by healthcare providers. The app would use algorithms that would provide patients with real-time recommendations for self-management of symptoms related to therapy. The companies also plan to investigate how it would allow patients to communicate more effectively with their providers, keep track of their symptoms and receive personalized care plans.
In a phone interview, Voluntis CEO Pierre Leurent declined to disclose which BMS drugs the partnership will focus on. “But this partnership is pretty broad, and our platform is applicable to a variety of assets,” he said. “The goal we have with this platform is to accommodate these different use cases.”
BMS has a diverse portfolio of oncology drugs, especially since its acquisition last year of Celgene. Key components of that portfolio include the PD-1 inhibitor Opdivo (nivolumab), the CTLA-4 inhibitor Yervoy (ipilimumab); and the immunomodulating drugs Thalomid (thalidomide), Revlimid (lenalidomide) and Pomalyst (pomalyst). Therapies in development include CAR-T cells like lisocabtagene maraleucel for non-Hodgkin’s lymphoma and idecabtagene vicleucel for multiple myeloma, the latter of which is under a partnership with bluebird bio.
Voluntis has existing partnerships focused on oncology with AstraZeneca and Novartis, as well as partnerships outside of oncology with AbbVie, Roche and Sanofi. The digital therapeutic it developed with AstraZeneca, eCO, is designed for women undergoing treatment for ovarian cancer in clinical trials of Lynparza (olaparib) combined with cediranib. Voluntis and AstraZeneca won a Prix Galien award in 2018.
Partnerships between biopharma and digital therapeutics companies have not all been smooth sailing. In October, Novartis terminated a partnership with Pear Therapeutics, while Proteus Digital Health and Otsuka Pharmaceuticals ended their partnership in January.
However, COO Romain Marmot pointed to the company’s success with CoaguChek Link – its partnership with Roche on coagulation – as demonstrating its experience with operating on a large-scale basis.
“We have had to face our share of challenges, but we have learned through these collaborations what works and what doesn’t,” Marmot said in the phone interview.
#voluntis#Bristol Myers Squibb#BMS#Digital Therapeutics#DTx#Digital Innovaiton#Health Innovation#Health Tech Startups#Cancer#Oncology
0 notes
Link
Ride-hailing titans Uber and Lyft have each launched a suite of new services aimed at addressing social determinants of health (SDOH), according to Forbes. For context, SDOH refers to health-influencing factors outside clinical settings — such as access to transportation — that drive 80% of health outcomes.

Here's an overview of the new services and what they mean for each ride-hailing giant's healthcare play:
Uber rolled out new patient-centric features, like multilingual notifications, opening up its nonemergency medical transportation (NEMT) services to a wider swath of patients. Uber's new features aim to improve the NEMT patient experience, and include enhancements such as designated pickup spots, multilingual notifications, and scheduling for landline users. These updates expand the ride-hailing titan's potential pool of users by making them available to patients who may have declined to use Uber due to cost or accessibility concerns in the past — such as the elderly or those without access to the Uber app or a smartphone.
Meanwhile, Lyft is diversifying its SDOH-focused offerings through its tie-up with social care coordination startup Unite Us. Lyft's partnership with Unite Us — a startup whose platform links healthcare organizations with social service providers — aims to make it easier for care providers to connect their patients with social services that impact an individual's overall health. For example, if a physician connects a patient with a job recruiter and that patient lands a job interview, Lyft could be called on to provide this patient with a ride. The tie-up with Unite Us enhances the scope of Lyft's SDOH-focused efforts by closing the gap between access to healthcare and access to social services — factors that both influence patients' health.
And as Uber and Lyft continue proving that their services can help reduce transportation and care costs, we expect to see both brands secure more provider and insurer tie-ups. CareMore Health System — which manages Medicare Advantage and Medicaid populations in select states — partnered with Lyft to enable caregivers to schedule rides for their patients and saw savings of more than $1 million in one year by switching from traditional transportation services, such as taxis.
And Boston Medical Center experienced a similar result, reporting $500,000 in transportation savings by leveraging Uber's NEMT services. As the ride-hailing titans continue proving the value of their services in reducing transportation costs, we expect to see insurers also pursue tie-ups with the likes of Uber and Lyft in an effort to ensure greater appointment adherence among their members. That's because payers will likely be looking for ways to slash their share of costs stemming from poor health outcomes due to missed appointments — which cost the US healthcare system $150 billion annually.
0 notes
Link
Virtual reality (VR) is making a difference on both sides of the doctor-patient relationship. Solutions for patients tend to focus on new treatment options for conditions like chronic pain and post traumatic stress disorder (PTSD). For doctors, virtual reality helps surgeons improve their skills and changes the training process for new doctors.
Healthcare's slice of the overall virtual reality and augmented reality market was $2.14 billion in 2018, according to a report from BIS Research. The company's "Global Augmented Reality and Virtual Reality Market in Healthcare – Analysis and Forecasts, 2019-2025" estimates that the market will reach $11.14 billion by 2025.
SEE: Managing AI and ML in the enterprise 2019: Tech leaders expect more difficulty than previous IT projects (TechRepublic Premium)
BIS Research reports that hardware and software are leading the way with 64.95% and 23.91% of the overall market, respectively. Extended reality--the term Accenture uses to describe "virtual reality, augmented reality, haptics, holograms and an expanding range of immersive tools" -- is also showing up in operating rooms.
This article highlights virtual reality services and products designed to treat patients and train doctors.
Practicing on virtual humans, not actual patients
Doctors and other healthcare providers need a lot of practice to master a standard medical technique, not to mention learn entirely new procedures required by new medical devices. When a surgeon or anaesthesiologist doesn't receive adequate training or enough practice, patient safety is at risk.
Medical Realities wants to use virtual reality modules to modernize physician training for students in medical school and practicing doctors. The healthcare tech company uses a SaaS model to provide immersive training for physicians.The Medical Realities platform covers a variety of training scenarios from a physician's office to the operating room to medical schools.
Based in the UK, Medical Realities plans to release its first product for college students in 2020. This course will allow undergraduates to watch and practice Objective Structured Clinical Examinations, which assess competency based on objective testing through direct observation.
Royal College of Surgeons and Samsung are both working with Medical Realities.
The Medical Realities app works with these VR platforms:
Gear VR
Google Daydream
Cardboard (Android)
Cardboard (iOS)
Oclulus Rift
HTC Vive
Osso VR is creating VR modules for surgeons, medical device sales teams, and hospital staff. The company's goals are to improve patient outcomes, increase the adoption of higher-value medical technologies, and democratize global access to the latest surgical techniques.
Founder Justin Barad has a background in software development, a bioengineering degree from UC Berkeley, and an MD from UCLA. The company offers custom content creation, a managed hardware leasing and support program, and performance analytics. Barad's background is orthopedics, and a validation study sponsored by the company focused on an ankle replacement technique.
In addition to training surgeons on the correct technique, Osso VR's simulations guide a doctor through the correct sequence of steps during a surgery. Another Osso VR product measures an individual's performance and progress as she completes the training modules.
Using VR to treat PTSD
Virtual reality experiences for patients offer a different kind of practice. Virtually Better creates virtual environments as a new way to provide exposure therapy. Psychologists use this method to create a safe environment where individuals can experience things they fear and avoid. Repeated exposure to objects, activities or situations in a safe environment can reduce anxiety in the long term.
The technologists and psychologists at Virtually Better have been developing virtual reality to treat mental health disorders for more than 20 years. The company works with mental health professionals to develop immersive virtual reality experiences to treat phobias, addiction, and even post traumatic stress disorder. Virtually Better has been developing Bravemind in collaboration with the USC Institute of Creative Technologies.
Bravemind has two virtual environments: Iraq and Afghanistan. Therapists can recreate difficult memories at a pace patients can handle. They do this by customizing the experience based on the patient's history to include explosions, firefights, insurgent attacks, and roadside bombs. The virtual experience includes sound effects such as weapon discharge and radio chatter, as well as vibrations designed to mimic engine rumbling and explosions. It also incorporates a scent machine to create smells appropriate to the setting, such as diesel fuel, garbage, and gunpowder.
Skip Rizzo is the director for medical virtual reality at USC Institute for Creative Technologies. In a video about Bravemind, Rizzo explains how the VR experience helps soldiers confront and process difficult memories.
"By this process of doing this repetitively over time, what you see is a gradual decline in the anxiety and fear response," Rizzo said. "We're not erasing memories, but those memories don't have the same intense painful emotional power that they had before treatment."
Based on learnings from Bravemind, the Virtually Better and USC teams are developing Strive, a virtual reality experience designed to build resilience and coping methods before soldiers are deployed.
Bravemind and Strive are being used in 50 VA hospitals and university medical centers around the country. Virtually Better works with universities including UCLA, the University of North Carolina at Chapel Hill, and the University of Houston.
MindMotion turns rehab into a game
Every year, more than 795,000 people in the United States have a stroke. This costs the United States an estimated $34 billion each year, including healthcare, prescriptions, and missed days of work.
Physical therapy is crucial for recovery, which typically includes motor-skill exercises, mobility training, and range-of-motion therapy. MindMotion products use virtual reality to turn these rehab routines into games.
MindMotion PRO is intended for hospital settings while MindMotion GO is used in physical therapy and rehab centers. Both have FDA clearance. The platforms include more than 30 neurorehabilitation gamified activities to keep patients engaged in treatment. For example, one module looks a lot like Fruit Ninja with the player holding a sword and swiping at floating fruit.
Therapists can customize the therapy based on the severity of the patient's condition, and the platform tracks progress over time. The PRO product can be used at the bedside, allowing patients to start rehab as soon as possible after a stroke. The GO product offers real-time audio and visual feedback so that both therapists and patients can track progress toward recovery. Early intervention improves the chances of a full recovery.
Rehab exercises are repetitive by nature, so setting the movements in the context of a game may increase patient engagement and adherence to therapy -- doing another round of exercises to boost your score is more motivating than working through a task list in the gym.
MindMaze is the parent company of MindMotion and was founded in 2012 as a spinoff from the Ecole Polytechnique Fédérale de Lausanne in Switzerland. MindMaze is headquartered in Lausanne, with offices in San Francisco, UK, Germany, France, and Romania.
How well does it work?
Researchers and physicians are still gathering information on how well these new treatments and training methods work. However, the demand for more doctors and more affordable healthcare show no signs of slowing down. If VR can help with either problem, it will become a tech investment with a high ROI.
0 notes
Link
Multiyear alliance underpins the Novartis commitment to leverage data & Artificial Intelligence (AI) to transform how medicines are discovered, developed and commercialized
Novartis to establish AI innovation lab to empower its associates to use AI across the business
Joint research activities will include co-working environments on Novartis Campus (Switzerland), at Novartis Global Service Center in Dublin, and at Microsoft Research Lab (UK) – starting with tackling personalized therapies for macular degeneration; cell & gene therapy; and drug design
youtube
Novartis today announced an important step in reimagining medicine by founding the Novartis AI innovation lab and by selecting Microsoft as its strategic AI and data-science partner for this effort. The new lab aims to bolster Novartis AI capabilities from research through commercialization and help accelerate the discovery and development of transformative medicines for patients worldwide.
As part of the strategic collaboration announced, Novartis and Microsoft have committed to a multi-year research and development effort. This strategic alliance will focus on two core objectives:
AI Empowerment. The lab will aim to bring the power of AI to the desktop of every Novartis associate. By bringing together vast amounts of Novartis datasets with Microsoft’s advanced AI solutions, the lab will aim to create new AI models and applications that can augment our associates’ capabilities to take on the next wave of challenges in medicine.
AI Exploration. The lab will use the power of AI to tackle some of the hardest computational challenges within life sciences, starting with generative chemistry, image segmentation & analysis for smart and personalized delivery of therapies and optimization of cell and gene therapies at scale.
Microsoft and Novartis will also collaborate to develop and apply next-generation AI platforms and processes that support future programs across these two focus areas. The overall investment will include project funding, subject-matter experts, technology, and tools.
Vas Narasimhan, CEO of Novartis, said, “As Novartis continues evolving into a focused medicines company powered by advanced therapy platforms and data science, alliances like this will help us deliver on our purpose to reimagine medicine to improve and extend patients’ lives. Pairing our deep knowledge of human biology and medicine with Microsoft’s leading expertise in AI could transform the way we discover and develop medicines for the world.”
Microsoft CEO, Satya Nadella, added, “Our strategic alliance will combine Novartis' life sciences expertise with the power of Azure and Microsoft AI. Together, we aim to address some of the biggest challenges facing the life sciences industry today and bring AI capabilities to every Novartis employee so they can unlock new insights as they work to discover new medicines and reduce patient costs.”
youtube
Novartis Data & Digital
Novartis is focusing itself as a leading medicines company powered by advanced therapies and data science. Going big on data and digital is a key strategic pillar that helps Novartis realize that ambition. Data science and digital technologies allow the company to reimagine how to innovate in R&D, engage with patients and customers, and increase operational efficiencies. Novartis focuses its efforts around four strategic digital priority areas:
Scaling 12 digital lighthouse projects: Build a strong foundation and jumpstart our digital transformation
Make Novartis digital: sharing, learning and talent acquisition
Becoming the #1 partner in the tech ecosystem: bridge Novartis with external expertise
0 notes
Link
Amazon just launched Amazon Care, a virtual primary care clinic with an option for nurses to visit employees in the home.
It’s described as a new benefit for employees that offers “the best of both virtual and in-person care.”
The company says it’s a pilot for employees in the Seattle area.
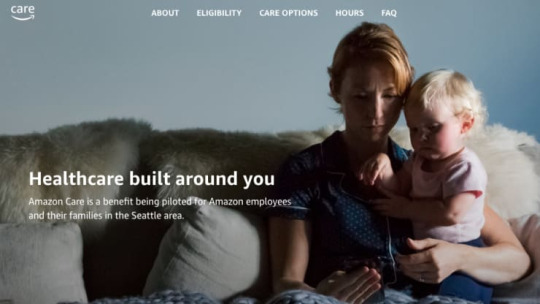
Amazon has launched a virtual health clinic with in-home follow-ups for employees in Seattle, dubbed Amazon Care.
The company announced the program on a web site, Amazon.care, that is currently publicly accessible but did not formally announce the news outside the company. “Amazon Care is a benefit being piloted for Amazon employees and their families in the Seattle area,” the website reads.
CNBC previously reported that Amazon was working on an employee health clinic for its Seattle-based employees. Those discussions kicked off last summer with a few hires, including a top Seattle doctor who ran a network of health clinics.
The clinic offers a combination of telemedicine and in-person services.
Its virtual offering includes an “in-app video visit with a doctor, nurse practitioner, or registered nurse ... for advice, answers, diagnosis, treatment or referrals,” according to the web site. Employees will have an option to see a health provider via a mobile app or website, and they can text a nurse on any health topic in minutes. If an employee needs follow-up care, Amazon Care can arrange for a nurse to pay a visit at home.
Amazon will also prescribe medications via Amazon Care within a few hours, or offer a way for employees to pick them up at a preferred pharmacy.
Health care represents a $3.5 trillion sector for Amazon, which is looking at ways to bring in technology ranging from cloud computing to medical record technology.
The company in 2018 joined up with J.P. Morgan and Berkshire Hathaway for an effort, later called Haven, to explore how to move the needle on health-care expenses, without compromising quality, for their combined 1.2 million employees. It has a pharmacy group under PillPack, a company it acquired in mid-2018, and a research and development group sometimes referred to as Grand Challenge or 1492. CNBC reported earlier this week that the company is working on wireless earbuds with some health and fitness-tracking features, representing its first foray into the wearables space, and could introduce them as early as Wednesday.
#Health Innovation#Digital Health#Amazon Health#Amazon Care#CNBC#Haven#Christina Farr#Employee Benefits
0 notes
Link
Best Buy Co. is well-known for bringing computers and other gadgets back to life. Now, it wants to take care of its shoppers’ health as well.
The retailer’s strategy to beef up its fledgling health-care business will be a key focus of its investor meeting Wednesday in New York. The plan includes selling everything from fancy fitness machines to health-monitoring services for seniors. It could help Best Buy grab some of the $3.5 trillion market for health spending in the U.S. -- while offsetting sluggishness in its main business of selling laptops, TVs and phones.
Best Buy Co. is well-known for bringing computers and other gadgets back to life. Now, it wants to take care of its shoppers’ health as well.
The retailer’s strategy to beef up its fledgling health-care business will be a key focus of its investor meeting Wednesday in New York. The plan includes selling everything from fancy fitness machines to health-monitoring services for seniors. It could help Best Buy grab some of the $3.5 trillion market for health spending in the U.S. -- while offsetting sluggishness in its main business of selling laptops, TVs and phones.
The health-care push is an opportunity for new Chief Executive Officer Corie Barry to put her own stamp on the company and step out from the sizable shadow cast by her predecessor Hubert Joly, who brought Best Buy back from the brink seven years ago. Barry is betting that Best Buy can thrive as digital-health technology migrates from hospitals to homes, but she’ll have to move fast since the retailer is just one of many companies looking to capitalize.
“I am not saying we will own the whole health-care experience -- we don’t want to,” Barry said in an interview. “But we are uniquely well-suited to be in people’s homes with technology. We can help with the tech side of health.”
Best Buy shares rose 1.5% to $68.41 at 9:42 a.m. in New York. The stock had gained 27% this year through Monday’s close, outpacing the increase of the S&P 500 Index.
Competition
Best Buy’s move into this space comes as Walmart Inc. has also stepped up its health-care ambitions, introducing low-cost medical clinics that offer primary-care services and also mental-health counseling. Another rival, Amazon.com Inc., paid $753 million for mail-order startup PillPack to get into the pharmacy business.
Best Buy wants to boost the revenue it gets from services like health care

Best Buy’s focus, meanwhile, is less on flu shots and more on the technology that underpins health-care services. There it faces challenges from tech giants including Google, Microsoft Corp. and Samsung Electronics Co., which are typically Best Buy’s partners.
The retailer has even hired a chief medical officer to spearhead it efforts, according to an internal memo obtained by Bloomberg. Daniel Grossman, a physician and veteran of medical-product maker Medtronic Plc who also practices at the Mayo Clinic, will join the company Oct. 1 and report to Asheesh Saksena, the president of Best Buy Health.
In the past year or so, Best Buy has spent upwards of $1 billion on acquisitions in the health space, most notably the $800 million purchase of GreatCall Inc., which sells mobile phones and emergency-response systems for older Americans. At the time, Joly called the senior market “white space waiting to be captured.” The number of Americans ages 65 and older is projected to nearly double to 95 million by 2060 from 52 million in 2018.
Best Buy followed up this year with two more tuck-in deals, buying senior-focused health-services company Critical Signal Technologies as well as a small engineering group in Watertown, Massachusetts, that designs wearable sensor systems that can help seniors live longer in their homes by predicting when they’ve fallen and need medical assistance.
“It’s all about Grandma,” Michael Pachter, an analyst with Wedbush Securities Inc., said in an interview. Because the company already is known as a seller of technology to consumers who need help figuring it all out, “it makes sense for Best Buy to offer this.”
The service part of the equation dovetails with Best Buy’s broader desire to expand its services revenue and lessen its dependence on products like video-game consoles, where demand can fluctuate and much-hyped gadgets can flop. The company now offers an annual service plan that promises to fix any product you own, no matter where it was purchased.
Services made up 6% of Best Buy’s U.S. revenue in its most recent quarter, up from 5% at the end of its last fiscal year. But it’s still a small slice of the overall business.
Simplicity, Trust
“People are spending less money on stuff,” Brian Owens, an analyst at Kantar Consulting, said. “Retailers need to invest in alternative revenue streams that provide services and experiences. As you evolve into health care, the big opportunity is simplicity. And people trust Best Buy.”
Products will also play into the strategy: Best Buy will put internet-connected fitness devices from brands like NordicTrack and ProForm into just under 100 stores in time for the holiday shopping season. Like other retailers, it also sells fitness trackers and products like the Owlet Smart Sock, which wraps around infants’ feet and tracks their sleep patterns and heart rates.
Not everything has worked so far, however. A Best Buy pilot program called Assured Living, which aimed to outfit seniors’ homes with a complex network of smart-home gadgets and sensors that caregivers could monitor, was deemed too difficult to sell in retail stores and has been reconfigured. Best Buy will also need to find more partners, like health-insurance companies, that can help promote the retailer’s offerings to their existing customers.
Investors will look for more details at the presentation, particularly a sense of how big this business can be -- and how much it can help offset any slowdown in electronics sales.
“We think the market doubts the durability of its core business,” Morgan Stanley’s Gutman said. “A next big thing is necessary.”
0 notes
Link
CANNES, FRANCE—As Lions Health wrapped up another year, attendees sifted their notes and pulled out a few common threads. Technology, for one, but not just for the sake of using a shiny new tool. Or as one Sanofi exec put it, "Technology in the service of creativity."
Marketing for good was another: aka, applying sharp communications brains to thorny public health problems. And then there were what Leigh Householder of Syneos Health called "secret messages," exemplified in one campaign using crossword puzzle clues to spread the word about elder abuse in a way abusive family members might not catch—but their puzzling seniors would.
Overall? It's a big world out there, but while appealing to global markets is key for pharma success, sometimes scaling up a creative local campaign is the way to really reach consumers and their doctors. Read on for more.
Tuesday
Doctors aren't robots. Forget the charts and graphs, sales reps. Lead with your heart instead. That’s the message from Attila Cansun, a chief marketing officer at the newly rebranded P&G Health. Adapting a consumer marketing framework dubbed LoveBrands, Cansun and his team use a similar evaluation-and-revamp for the company’s doctor relationships. Story
The trust tour. GW Pharmaceuticals' strategy for promoting its new cannabinoid drug Epidiolex to doctors sounded simple—earn their trust. But that's easier said than done, no thanks to Kim Kardashian and her CBD-themed baby shower. So the company decided to take physicians to the source, namely its massive marijuana greenhouse, and show them cannabis-derived drugs are the real deal. Story
Get out the tissues. In the U.S., when seasonal sneezes come on, there’s no mystery about what to do next: Go to the drugstore for allergy medication. But what if that decision weren’t automatic? For Sanofi, as it launched its drug Allegra over the counter in Brazil, the answer was a mobile, augmented-reality campaign that could outfit users with red noses and watery eyes, similar to a Snapchat filter. It even animated sneezes, complete with droplets on the phone screen. Story
Hemophilia, humor and a hipster host. A reality show about hemophilia? And it's a comedy? That would be Roche’s Genentech and ad agency 21 Gram’s new YouTube series called “Challenge Accepted.” Each 15-minute episode of the show, which premiered last week, features a different patient, coach and lesson targeting boys and young men with hemophilia. And it gets that lesson across using humor—and some magic tricks. Story
Monday
Big winner. It’s been a long three years since a pharma jury here handed out Grand Prix honors. But Monday night, GlaxoSmithKline and its ad agency McCann Health broke through with a mobile app called Breath of Life, used to detect chronic obstructive pulmonary disease in older adults in China. Eli Lilly, ViiV and Merck were among the other pharma winners. Story
No-ad advertising. Marketers need to think big. Like saving-the-world big. That’s the advice McCann Health’s global chief creative officer, Matt Eastwood, offered up to kick off the Cannes Lions Health confab Monday. And what that means to Eastwood is advertising that “may not look like advertising,” he said. “It’s advertising while doing good.” Story
New light on MS. Multiple sclerosis symptoms can be confusing: They differ person to person and even day to day for the same patients. Roche's Floodlight Open app aims to clear that confusion for individuals by gathering personal health data every day and delivering real-world evidence for broader understanding of the disease. Story
Procter & Gamble showed up for Cannes Lions Health—and no wonder. The consumer packaged goods giant recently bought Merck KGaA’s consumer health division and rebranded it as P&G Health. Now, with teams of brand managers and concept demos on display here, it's serving notice—at least to consumer healthcare companies—that it’s in play. Story
Sunday
Shorted shortlist? The Pharma Lions shortlist for 2019 lives up to its name. Just 31 entries cleared the finalist bar this year, down from 53 last year. But it’s still good news for the 12 multinational pharmas in the running—and for the pharma marketers looking to eye the industry’s best at Monday night’s awards. Story
Breakout scouts. As Cannes Lions Health kicked off its sixth year Monday, attendees were looking for some breakouts. As in breakthrough technology, creativity that breaks barriers, groundbreaking global ideas and a chance to finally break with the backhanded compliment that Lions Health work is “good for pharma.” Story
Escaping the same-same. Bartle Bogle Hegarty’s roster of literal A-list clients—Audi, Absolut and AstraZeneca—earns it center stage at Lions Health with a consumer-to-pharma creative crossover pitch. Hint? Brand differentiation is job one, and to do that, drugmakers need to pull cliché imagery out by the root. Story
Heard around the Palais
Winning outside the pharma and healthcare box. Johnson & Johnson and partners picked up the Grand Prix for Entertainment on Tuesday night for the film “5B.” The film about the nurses who founded and opened the first AIDS ward at San Francisco General Hospital, called Ward 5B in 1983 was picked up in May by Verizon for broader distribution and officially debuted June 14.
“5B” was created by Oscar-nominated director Dan Krauss and Saville Productions, known for its branded ad and film work. Saville tweeted in May after Verizon picked up “5B”: “The film is an HIV/AIDS crisis doc, commissioned by Johnson & Johnson, & shows how brands can authentically get behind global issues.” The film also won a Lions Health & Wellness silver statue on Monday night.
Stand up for nontraditional creative. Getting the green light for a brand film targeted at physicians at a pharma company is not for the faint of heart. Even the best ideas face multiple hurdles. AstraZeneca’s Lions Health award-winning "The Attack" film from last year highlighted the risk of repeat heart attacks, but was "that close" to being killed, said Kyriakos Konstantinidis, global strategy director at AstraZeneca. That's what his boss told him later after winning four more global awards and industry impact, feedback and successes rolled in. It helped that Konstantinidis understood the vision that ad agency Havas Lynx brought with its ideas—his father had worked in the film industry and he grew up in and around it. Konstantinidis and Havas' perseverance not only led to the creation of "The Attack," but another new disease awareness film about cardiovascular risks around Type 2 diabetes that debuted recently. That patient-targeted film features a fisherman talking about the dangers of heart disease for Type 2 patients while his boat engine stalls, noting, "That's how dangerous Type 2 diabetes is. It can cause your heart, your engine, to fail." Konstantinidis urged the audience and pharma in general to take risks, albeit calculated ones with a strategy meets creativity foundation. "When you're working in the pharmaceutical industry which has a very conservative approach to these sort of ideas, and you have your colleagues and peers coming to you (after the campaign) to say, apart from congratulations, 'How did you do that? We want to copy that, we want to do the same brief. ... What is the direction I need to take internally to make this a success in order to get the buy in?' Then you know you have created a new area," he said.
Fighting fake news. Panelists from Johnson & Johnson, McCann Health and the Centers for Disease Control and Prevention tackled the thorny problem of combating disinformation about vaccines. "It won't be a TV campaign," said Seema Kumar, J&J's vice president for innovation in global health and science policy. Social media might play a role, but it's really peer-to-peer and face-to-face that's most important, the panelists decided. How to make that happen? "I'm challenging you," panel moderator Rajesh Mirchandani, chief communications officer of the UN Foundation, told the audience.
In another session moderated by Conde Nast’s health chief Jen Mormile, Self editor in chief Carolyn Kylstra said their research found that while fake health news is not new—snake oil has been around a long time—it’s an issue because of social media and the rapid way disinformation now spreads, exacerbated by the lack of trust in authority figures. To combat it, Self is doubling down on health news editing, fact checking and vetting sources including celebrities. Meanwhile, co-panelist physician Esther Choo said doctors can do their part by going beyond traditional journal model of publish-and-done model. Today physicians need to follow up and make sure other scientists are misusing or misconstruing their work, she said.
VR for VR's sake. Too many pharma marketers are making the mistake of recruiting tech tools for jobs they can't do, or just using those tech tools poorly, said Sam Glassenberg, founder and CEO of LevelEx, which makes mobile games for physician training. For example, method-of-action presentations that used to be on big screens have moved into virtual reality. But zooming around a molecule in the virtual world while sitting still in the real one tends to make people nauseous. "The last thing you want a doctor to associate with your drug is nausea," Glassenberg said. The moral of the story is to use VR only when it's the only way to tell a particular story—and when you do, make it a spectator sport, he said.
Real-world evidence and technology are natural partners, said Alex Gilbert, head of partnerships at app developer Medopad, as he ticked off the ways his company’s working with life sciences companies such as Novartis to help collect the sort of patient data that can help drugmakers add new indications to existing drugs. “We’re now creating a living, breathing document of a patient," Gilbert said. “Your health record doesn’t have to be in the hospital. It’s here, created by you.”
Spending on real-world evidence has hit $1.48 billion, according to Simone Seymour, founder and CEO of the biometric textiles company Supa. Real-world evidence is “really hot right now” for personalizing products but “also for understanding how drugs are actually working.” Companies are tapping Supa’s tech—and its network of app users—to monitor and recruit patients for real-world evidence trials.
A GlaxoSmithKline toothpaste made to alleviate bleeding gums served as an example of why design should be at the heart of marketing in one session led by GSK's vice president of design and innovation Andrew Barraclough. "It's all about spitting blood," he noted, and blood imagery appeared on everything from the platelet-like motif on the toothpaste tube to the smartphone screen that started bloody and cleared from there. Then, in the Czech Republic, GSK flipped the idea to promote blood donations. "Don't spit blood, give blood" was the tagline for GSK's #bloodforgood campaign there.
0 notes
Link
Dr. David Feinberg has spent his entire career caring for people’s health and wellbeing. And after years in the healthcare system, he now leads Google Health, which brings together groups from across Google and Alphabet that are using AI, product expertise and hardware to take on big healthcare challenges. We sat down with David to hear more about his pre-Google life, what he’s learned as a “Noogler” (new Googler), and what’s next for Google Health.
You joined Google after a career path that led you from child psychiatrist to hospital executive. Tell us how this journey brought you to Google Health.
I’m driven by the urgency to help people live longer, healthier lives. I started as a child psychiatrist at UCLA helping young patients with serious mental health needs. Over the course of my 25 years at UCLA, I moved from treating dozens of patients, to overseeing the UCLA health system and the more than a million patients in our care. Then, at Geisinger, I had the opportunity to support a community of more than 3 million patients.
I recall my mom being very confused by my logic of stepping away from clinical duties and moving toward administrative duties as a way of helping more people. However, in these roles, the impact lies in initiatives that have boosted patient experience, improved people’s access to healthcare, and (I hope!) helped people get more time back to live their lives.
When I began speaking with Google, I immediately saw the potential to help billions of people, in part because I believe Google is already a health company. It’s been in the company’s DNA from the start.
You say Google is already a health company. How so?
We’re already making strides in organizing and making health data more useful thanks to work being done by Cloud and AI teams. And looking across the rest of Google’s portfolio of helpful products, we’re already addressing aspects of people’s health. Search helps people answer everyday health questions, Maps helps get people to the nearest hospital, and other tools and products are addressing issues tangential to health—for instance, literacy, safer driving, and air pollution.
We already have the foundation, and I’m excited by the potential to tap into Google’s strengths, its brilliant people, and its amazing products to do more for people’s health (and lives).
I believe Google is already a health company. It’s been in the company’s DNA from the start.
This isn’t the first time Google has invested directly in health efforts. What has changed over the years about Google’s solving health-related problems?
Some of Google’s early efforts didn’t gain traction due to various challenges the entire industry was facing at the time. During this period, I was a hospital administrator and no one talked about interoperability—a term familiar to those of us in the industry today. We were only just starting to think about the behemoth task of adopting electronic health records and bringing health data online, which is why some of the early projects didn’t really get off the ground. Today we take some of this for granted as we navigate today’s more digitized healthcare systems.
The last few years have changed the healthcare landscape—offering up new opportunities and challenges. And in response, Google and Alphabet have invested in efforts that complement their strengths and put users, patients, and care providers first. Look no further than the promising AI research and mobile applications coming from Google and DeepMind Health, or Verily’s Project Baseline that is pushing the boundaries of what we think we know about human health. And there’s so much more we can and will do.
Speaking of AI, it features prominently in many of Google’s current health efforts. What’s next for this research?
There’s little doubt that AI will power the next wave of tools that can improve many facets of healthcare: delivery, access, and so much more.
When I consider the future of research, I see us continuing to be deliberate and thoughtful about sharing our findings with the research and medical communities, incorporating feedback, and generally making sure our work actually adds value to patients, doctors and care providers.
Of course, we have to work toward getting solutions out in the wild, and into the hands of the pathologist scanning slides for breast cancer, or the nurse scanning a patient’s record for the latest lab results on the go. But this needs to be executed safely, working with and listening to our users to ensure that we get this right.
Now that you’ve been here for six months, what’s been most surprising to you about Google or the team?
I can’t believe how fantastic it is to not wear a suit after decades of formal business attire. When I got the job I ended up donating most of my suits. I kept a few, you know, for weddings.
On a more serious note, I’m blown away every day by the teams I’m surrounded by, and the drive and commitment they have for the work they do. I’m thrilled to be a part of this team.
What's your life motto?
I know this sounds cheesy, but there are three words I really do say every morning when I arrive in the parking lot for work: passion, humility, integrity. These are words that ground me, and also ground the work we are doing at Google Health.
Passion means we have to get this right, and feel that health is a cause worth fighting for, every day. We need humility, because at the end of the day, if we move too quickly or mess up, people’s lives are on the line. And integrity means that we should come to work with the aim of leaving the place—and the world—better than when we found it.
0 notes
Link
Healthcare Chatbots market has shown strong growth pattern in recent year’s majorly due to growing internet connectivity and rising adoption of smart devices, limited availability to council huge patient population, technology advancement in artificial intelligence, chatbots to reduce the healthcare cost, and increasing demand of virtual health assistance. In addition, social media supporting chatbot platforms, and integration of chatbots with the IOT further boosts the growth of this market during the forecast period.
Meticulous Research® in its latest publication on healthcare chatbots market states that the global healthcare chatbots market will increase at a CAGR of 25.1% from 2019 to 2025 to reach USD 703.2 million by 2024. Wherein, geographically, Europe commanded the largest share in this market followed by North America. The major share of Europe is mainly attributed to rise in awareness, investments in AI technology, conducive investment environment for AI startups working on development of AI-powered chatbots, and growing adoption of chatbots among healthcare providers.
Request Sample Report: https://www.meticulousresearch.com/request-sample-report/cp_id=4962
The report provides meticulous analysis of global healthcare chatbots market by segmenting it on the basis of component (software, services), mode of deployment (on premises, cloud), application (symptom checking, medication assistance & guidance (consultation), coverage & claims management, appointment scheduling), end user (patient, healthcare providers, healthcare payers). Wherein, among mode of deployment, on premise segment commanded the largest share in this market. The largest share of this segment is primarily attributed to ease of implementation and minimizing data breach.
The key players analyzed in the global healthcare chatbots market are Infermedica, Sensely, ADA Digital Health, Babylon, HealthTap, Your.MD, Baidu, Buoy Health PACT Care, Woebot Labs, and GYANT.
Browse in-depth Report on https://www.meticulousresearch.com/product/healthcare-chatbots-market-4962/
Key Topics Covered in This Report:
1. Introduction
1.1. Market Definition
1.2. Market Ecosystem
1.3. Currency and Limitations
1.3.1. Currency
1.3.2. Limitations
1.4. Key Stakeholders
2. Research Methodology
2.1. Research Process
2.1.1. Secondary Research
2.1.2. Primary Research
2.1.3. Market Size Estimation
3. Executive Summary
3.1. Introduction
3.2. Market Analysis, by Component
3.3. Market Analysis, by Mode of Deployment
3.4. Market Analysis, by Application
3.5. Market Analysis, by End User
3.6. Regional Market Analysis
4. Market Dynamics
4.1. Introduction
4.2. Drivers
4.2.1. Growing Internet Connectivity and Rising Adoption of Smart Devices
4.2.2. Limited Availability of Healthcare Resources to Address Growing Patient Population
4.2.3. Technology Advancements in Artificial Intelligence
4.2.4. Chatbots to Reduce Healthcare Cost
4.2.5. Increasing Demand of Virtual Health Assistance
4.3. Market Opportunities
4.3.1. Social Media Supporting the Chatbots Platform
4.3.2. Integrating Chatbots with the Iot
4.4. Market Challenges
4.4.1. Data Privacy Concerns
4.4.2. Lack of Expertise for Chatbot Development
4.4.3. Lack of Awareness and Misconceptions
5. Global Healthcare Chatbots Market, by Component
5.1. Introduction
5.2. Software
5.3. Service
6. Global Healthcare Chatbots Market, by Mode of Deployment
6.1. Introduction
6.2. On-Premise
6.3. Cloud-Based
7. Global Healthcare Chatbots Market, by Application
7.1. Introduction
7.2. Symptom Checking
7.3. Medication Assistance & Guidance (Consultation)
7.4. Coverage & Claims Management
7.5. Appointment Scheduling
8. Global Healthcare Chatbots Market, by End User
8.1. Introduction
8.2. Patients
8.3. Healthcare Providers
8.4. Healthcare Payers
9. Global Healthcare Chatbots Market, by Region
9.1. Introduction
9.2. Europe
9.2.1 UK
9.2.2. Germany
9.2.3. France
9.2.4. Rest of Europe (ROE)
9.3. North America
9.3.1. U.S.
9.3.2. Canada
9.4. Asia Pacific (APAC)
9.5. Rest of the World
10. Competitive Landscape
10.1. Introduction
10.1.1. Market Ranking Analysis
10.1.2. Growth Strategy Analysis
11. Company Profiles
11.1. Your.MD Limited
11.2. Baidu, Inc.
11.3. Sensely, Inc.
11.4. Babylon Healthcare Services Limited
11.5. HealthTap, Inc.
11.6. Buoy Health, Inc.
11.7. Infermedica
11.8. Ada Digital Health, Ltd.
11.9. PACT Care BV
11.10. Woebot Labs, Inc.
11.11. GYANT.COM, INC.
12. Appendix
12.1. Available Customization
12.2. Questionnaire
Download free sample report here: https://www.meticulousresearch.com/download-sample-report/cp_id=4962
0 notes
Link
Healthcare is in the midst of a digital shift. Artificial intelligence offers physicians providers an new resource when making decisions, new data streams promise hyper-personalized treatments and digital therapeutics evangelists see are hawking the novel treatments as a companion, or even a replacement, to traditional biologic treatments. All in all, it’s a future that hopes to empower the individual while streamlining care and cutting costs — but only if pharma and other established players can identify its challenges and properly embrace what’s to come.
“The ecosystem has shifted, and it’s put more power into the hands of people who give the data, more power into the hands of the patients,” Dr. Ameet Nathwani, chief medical officer and chief digital officer at Sanofi, said yesterday to a packed room at BIO 2019 in Philadelphia. “[That] is a good thing, but we in the industry have to find out what is our role in that journey.”
Avoid mistakes from the past
Nathwani said that pharma has often fallen short with most promising innovations because it did not consider the ecosystem into which they would be introduced — take, for instance, the industry’s development and launch of therapies that required multi-hour infusions prior to infusion clinics becoming commonplace in health systems. The modern equivalent, he explained, is uncertainty as to whether or not the biologic treatments currently undergoing clinical trials will still make sense within the data-driven care ecosystem to come.
“Ask yourself, ‘Are people treated the same way that they are in your trial by the time the drug is actually approved and priced in the markets?” Nathwani advised. “Try and predict within three or four years after your product is available, how are people going to be treating their disease, how are patients expecting to be managed, and is that the same way that you’re developing your drug. Because if it isn’t, more people are going to ask the question: Well, this is great, you did your trials in five or six years in a very traditional manner, but in those five or six years … [patients] managed at home, there’s digital biomarkers, there are new devices that are probably used, the patient’s journey is probably completely different. If you have a more traditional approach, will your data still fit in that new model? I would ask yourself those questions now, and have those scenarios built in as you develop your drugs.”
Another consideration is growing presence of AI in everything from drug development to clinical decision support, he continued. On the one hand, there’s no reason for pharma not to lay the groundwork for tools that can address its biggest drivers of cost and inefficiency.
“My first message to everyone who is in our industry right now is to use technology to enable your trials, your conventional work,” he said. “We should be looking at that — how do we find patients, how do we codify them, how do we do the trials a completely different way? The technology is not there today, but we should maybe look at conducting trials more efficiently because the cost of R&D is still huge across our industry, and I think that’s ripe for disruption."
Much like other industries, however, healthcare is not immune to the broader threat AI and automation bring to the traditional workforce. Even beyond careful data governance, thoughtful system design and other efforts toward building responsible and transparent AI, Nathwani said it was important for pharma to begin preparing its people for the culture shift.
“The question is, how do we retool a generation to become more data driven, much more oriented to that,” he said. “We have a big program that we just kicked off internally to get our organization digitally literate to start with, and then we have to focus on building new capabilities. We certainly cannot hire everyone we need to, there’s already a shortage in the world of data engineers and data scientists … It’s a major problem, I don’t have a solution. It’s a society problem as well.”
Friend, not foe
And then there’s the question of the new kid on the block: digital therapeutics. For Nathwani and Sanofi, the maturing sector doesn’t necessarily represent a threat to the traditional business of biologic-based medications. Rather, he said, pharma companies should be carefully watching these smaller digital companies for signs of which parts of its business could be disrupted or replaced.
“We just have to be mindful. It’s the same as any other competition coming in, except it’s just very different and we have to have a different mindset,” he said. “A digital therapeutic can be a friend or a foe, we just have to open our eyes to the fact that competition is coming from new angles that we otherwise would not have considered. Our approach and response should be equal: Let’s embrace it, understand what it can do. If the patients are going to benefit in the end, that’s great for everyone. If we can’t differentiate our drug from a digital therapeutic we have a different problem, because I think digital therapeutics will be faster, they’ll be quicker, they won’t have the same safety burdens that you would encounter with our own drugs.”
That’s not to say that adopting these new modalities will be simple. While many digital therapeutics lack the adverse events of biologics and therefore may seem low risk to develop, there are are a number of logistic and regulatory unknowns that won’t be easy to untangle.
“The number one [risk to pharma] is just the complexity of developing these technologies,” he said. “Just imagine, when you develop a biologic drug and you make a slight modification, you have to go through this whole process. Just imagine that in the software world, where software changes over time. What’s the IP of it, can you IP a software? Well, actually, the regulations are not that clear and so there’s software’s copyright, and then what’s the generic version of it?”
Again, Nathwani said that pharma companies need to put more effort into investigating and anticipating which digital therapeutics or tools will drive the paradigm shift. To do so, he suggested that organizations should take a deeper look into the platforms being developed by tech companies in and out of the immediate space and, if it makes sense, follow their lead.
“There’s a number of tech companies working in this space. Take a scan of it and see how many other people are actually interested in those disease areas and see what they’re doing,” he said. “We’re very good at scanning [for] other biological competitors and understanding who’s developing similar products and similar drugs in the same ecosystem. We’re not very good at anticipating what are the technologies that are being developed that could fundamentally shift the ecosystem.”
Cutting costs, providing relief
Beyond simply keeping up with the changing landscape, Nathwani said that preparing for and embracing these new patient-friendly technologies is all but mandatory to combat ballooning price tags. As the cost of managing chronic conditions and delivering treatments is threatening to prohibit the development of novel treatments, he explained, any opportunity for pharma to remove costs from the system can’t be overlooked.
“I’m not sure that digital health is going to solve the fact that pharma is still regarded as just above the tobacco and oil industry in reputation. But I think what we do have is a responsibility right now … to actually manage chronic diseases more effectively, and more cost effectively,” he said. “Do you want to see a world where genetic therapy or cell-based therapies cannot be delivered? I think in order to afford that, we have to find a redress in the balance, take healthcare costs out in other ways, and be responsible in doing that. … And we can’t control everything — there’s also other infrastructure costs we can’t control — but at least we can play a role in that, and I think digital health is one way that can help us address the balance.”
0 notes
Link
In 1993, Ruth Riechl, the new restaurant critic for the New York Times, penned a memorable review of Sirio Maccioni’s elegant Manhattan restaurant Le Cirque (which closed, at least temporarily, in January 2018). Riechl described two distinct experiences she had at the establishment, first as an anonymous diner, then as a recognized Times food critic; in the first instance, she received a bad seat after a long wait, was treated rudely, then served food that was (relatively) mediocre. But once she was recognized as a VIP, she was duly treated like royalty – felicitous seating, solicitous service, and sublime food. In presenting these experiences together, Riechl highlighted both the typical meal experience of most diners as well as the transcendent experience that was possible. (I went to Le Cirque in the mid-80s to celebrate my high school graduation, in my pre-low-carb days; while I can’t remember where we sat, the food, particularly the legendary potato-wrapped bass, was delicious).
It occurred to me that in many ways, innovation at large pharmas can be experienced very similarly – so often, disappointing and stifling, but occasionally, under the right circumstances, transformative and elating.
This dual-nature of pharma innovation may explain both why so many innovators are repelled by large pharma companies, yet some – including those focused on digital and data – are deliberately seeking out opportunities in these corporations.
In contrast to big drug companies, the appeal of startups is easy to understand – the self-actualization, the sense that your individual contribution not only matters but is essential, the feeling of David vs Goliath, the allure of significant upside, both in terms of impact (disruption, making the world a better place, etc.) and financial return. You can really get a good feel for this by watching the HBO Theranos movie, The Inventor, where you can see how so many people were drawn to the startup for this powerful combination of reasons. According to this documentary at least, Theranos offered all these elements, lacking only an actual, functional product and an achievable plan to create one. (My thoughts on Carreyrou's Theranos book, Bad Blood, are here.)
What’s interesting to me is the increasing number of well-trained, innovative people I seem to be running into, particularly on the digital and data side, who are coming to large companies after spending time in health tech startups, not because they’ve somehow given up on their dreams, but rather because, in some ways, they’re more serious about them, and are seeking more than the superficial accouterments of tech startups (so brutally described in Disrupted, by Dan Lyons). Moreover, these innovators are joining large companies with eyes wide open; they recognize the very real, and highly problematic challenges large companies have with agility and decision-making. Nevertheless, it seems like these innovators (at least the few I’ve met) hunger for the chance to really make a difference in the application of tech to health and drug discovery and development, to work towards a result not twitter-worthy but FDA-worthy, in the context of a well-resourced and credible organization capable of responsibly delivering it.
(Disclosure/reminder: as a corporate VC, I arguably have a foot in both pharma and startup camps.)
The Bad News
First, the bad news. The equivalent to entering Le Cirque as an anonymous patron in 1993 is joining pharma and trying to innovate against the grain. Everything is arrayed against you.
Large organizations tend to be remarkably risk-adverse, essentially because they have an established, successful enterprise and generally worry more about the downside risk of any given opportunity then the upside possibility it could represent. The implicit calculation is pretty simple: one screw-up could bring the whole organization down, while one striking success is unlikely to move the needle all that much. In contrast, startups tend to have very little to lose, and if they’re lucky and/or good, a lot to gain – hence their view of risk is quite different.
To be sure, in most large organizations, no one wants to inhibit innovation -- at least not explicitly. Innovation, like failure, is something to publicly cherish and visibly celebrate – the kind of thing that’s abstractly good for an organization to value, but generally not needed or welcome in your operational group, where you’re already plenty busy trying to get defined tasks completed, thank you very much.
But even if you’re skeptical about innovative proposals, to operate successfully in large, highly matrixed organization, you need to maintain generally cordial relationships with as many people as possible. The result is what I first wrote about in 2011, when a senior pharma executive who had recently transitioned to industry from a top Harvard hospital remarked to me that:
“his greatest shock upon joining the business world, the thing he was least prepared for, wasn’t the business vocabulary, the timelines, the quarterly expectations of wall street analysts – none of the above. Instead, it was dealing with the passive aggressive behavior he discovered everywhere around him.”
It’s a phenomenon I’ve described as “innovation dissipation,” where no one explicitly says “no” to a new idea, it just winds up ping-ponging through an organization until it eventually peters out.
Recently, a colleague offered what I thought was an astute explanation for this phenomenon: “Why spend political capital saying ‘no?’” he asked me. He’s right. The savviest, most senior players in complex organizations seem especially adept at this, politely taking meetings and pursing their lips while listening thoughtfully, and then suggesting several follow-up meetings they know full well aren’t likely to lead anywhere.
It turns out, there’s even a phrase for this mindset: “trust the process.” This may not have started out as cynical in spirit, but in practice, in a large organization, it basically means let the process play out, and don’t try to rock the boat by interfering. The result – as Safi Bahcall brutally describes in Loonshots (my WSJ review here, and my more detailed discussion of this exact point here) – is a culture where everyone is highly attuned to the (perceived) views of those at the apex of the hierarchy, and original, orthogonal, or non-incremental perspectives will struggle to be heard. That’s the system, and often the fate of bottom-up innovation within it.
At this point, would be innovators out there might be ready to don their Allbirds, sling their Herschel backpacks over their shoulders, grab their Sightglass lattes, and head off the to closest WeWork.
Not so fast. I’ve recently spoken with several health tech innovators who actually did something more or less like this early in their careers, then quite deliberately choose to take their talents to large pharma companies with many of the liabilities enumerated above. What were they thinking?
The Good News: The Three Rs
Turns out that, like VIPs dining at Le Cirque, innovators who find themselves aligned with and integrated into pharma strategy may be treated to an exceptional experience. According to several such well-situated innovators, large, incumbent companies have a lot going for them; in particular: resources, redundancy, and results.
The resource aspect is fairly obvious: when a large company truly commits to a particular strategy, approach, or technology, they are able to pursue this goal in a deep, remarkably thorough way, deploying people, capital, and leveraging (as well as acquiring) institutional know-how. Example: a few months ago, I heard a senior pharma oncology leader describe the way they were approaching a particular category of high-priority targets, and it was mind-blowing in scope, staggeringly comprehensive. Multiple options were systematically evaluated at almost every step in the process – truly the “mutually exclusive, collectively exhaustive (MECE)” concept applied to a particular area of biological discovery. Offerings from many startups were considered at each of these stages, and it was hard not to be struck by the observation that while a small company could potentially optimize one particular solution or approach, the large pharma could effectively afford to choose from among these to pick the best one.
The second, often underappreciated aspect that several innovators kept returning to is the redundancy and depth you see in big pharma; I was regaled with stories of how, in startups, you often have only a single person in a key area like legal or regulatory, and you are disproportionately dependent on their expertise, not only in terms of what they know, but also their ability to recognize their own gaps. Obviously, there is a huge emphasis in startups in hiring excellent people, but in many ways, startups operate largely without a net, a precarious situation which can, and often does, prove disastrous to young companies.
The last, and in some ways most important difference between startups and large companies is that at the end of the day, many startups just need to look promising enough to justify an acquisition or an IPO – sizzle with the promise of steak. But at a large company, the buck stops with you in many ways; your business depends not on the glamor or glitz of an emerging technology, but on actually getting it to work, and getting it to market. Thus a buzzy startup like Stemcentrx could make billions for its investors, yet ultimately fail in the hands of the pharma company who acquired it and tried to bring the products to market. The jury still seems to be out for the early CAR-T companies (including Juno, acquired by Celgene [itself acquired by BMS], and Kite, acquired by Gilead). (Disclosure: my wife works at Gilead though not in oncology.)
Just as academia tends to attract researchers who pursue novel science, and biotech startups often attract researchers keen to turn raw science into promising therapeutics, pharma attracts many researchers with the determination and patience to see raw science and promising therapeutics through to approval and into the clinic. Their mission is achieving clinical impact at scale, and it’s a powerful draw for some innovators.
Bottom Line
Pharmas are attractive for innovators pursuing approaches that are strongly endorsed by senior leadership and reasonably welcomed by the operational areas of the organization. The way some pharmas are working through the complex supply-chain logistics required for delivering CAR-T therapy or gene therapy at scale offer striking examples.
On the other hand, pharma organizations generally prioritize caution over agility, and incremental change over radical new approaches. Thus even innovation welcomed by the C-suite (like a lot of the original digital and data efforts) can run into the grindstone when those in the trenches can’t see the benefit, and experience only burden.
In general, large pharmas, like other big companies, are likely to remain generally resistant to profound innovation, though they will embrace and really go after specific opportunities they view as adequately validated or promising. Such traction requires explicitly endorsement and constant, active support from the top echelons of management if the approach is to even have a chance. Meanwhile, detached innovation initiatives reliably garner transient publicity but tend to achieve little durable organizational impact.
There’s likely a considerable opportunity to harness the many bottom-up innovative ideas to which pharma seems constitutively unable to respond; the robust startup ecosystem offers an attractive alternative or salvage pathway for some but not all of these promising approaches.
0 notes