#ThermodynamicThursday
Text
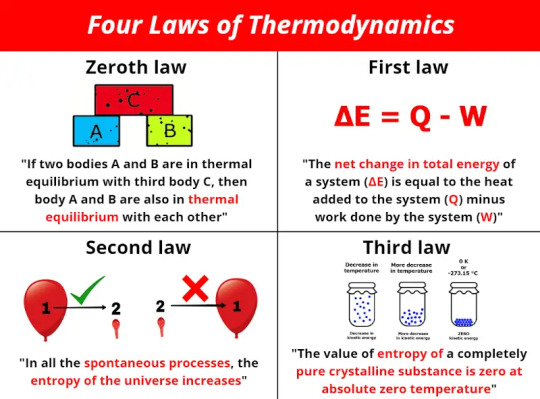
Thermodynamics
Thermodynamics is defined as a branch of physics that deals with energy, primarily heat, temperature, and the work of a system. More specifically, it deals with the relationships between these values and others, including entropy and the conversion of one form of energy to another. Only large scale, relatively speaking, system responses are defined as thermodynamics - the overall trend or reaction a system exhibits. There are four laws of thermodynamics, defined in the image above.
Sources/Further Reading: (Image source - LinkedIn) (Wikipedia) (NASA) (Harvard Lecture) (Livescience.com)
90 notes
·
View notes
Photo

Remembering #CircuitCity by @moormother at @fringearts - a choreoplay / Black quantum futurist exploration of public/private ownership, housing, and tech set in a living room in a corporate owned apt complex | #phillyfringe #afrofuturism #theater #blackquantumfuturism #housingfutures #communityfutureslab #blackwomenartists #phillyfringe #tbt #thermodynamicthursday #timemachine #housing #diytimetravel (at FringeArts) https://www.instagram.com/p/B3vbzuMlSlm/?igshid=1olmvqoq3j5t2
#circuitcity#phillyfringe#afrofuturism#theater#blackquantumfuturism#housingfutures#communityfutureslab#blackwomenartists#tbt#thermodynamicthursday#timemachine#housing#diytimetravel
0 notes
Note
I have a question, can ice dissolve in liquid oxygen?As oxygen can dissolve in water please
Woo hoo, it’s a thermodynamic thursday!
Let’s start by thinking about these fluids the other way. Under standard temperature and pressure, water will be a liquid and oxygen will be a gas. Oxygen doesn’t dissolve well in water; they’re two very different kinds of molecules. H2O is strongly polar, with one side positively charged and another side negatively charged. That’s why water dissolves salt so well - it splits the salts into ions and the positive ions attract the negative side of the water molecule and vice-versa. Oxygen is a non-polar, covalently bonded molecule, with no strong charge on either side. Therefore, oxygen does not dissolve well in water - there isn’t enough oxygen in water for my lungs to work if you fill them with water; I drown. However, there is a tiny bit of oxygen that will dissolve in the liquid. This amount of oxygen is much less concentrated than in the air, but animal life has figured out how to use it (hence: fish exist). Small amounts of non-polar molecules will still dissolve in a solution of polar molecules like water. To simplify a bit, this comes down to entropy; there is an energy benefit to having a tiny bit of other “stuff” dissolved in a solution rather than having a pure solution because it increases the entropy of the system. So, oxygen does dissolve in water even though it doesn’t fit in there chemically, and that’s enough for fish to exist.
Now turn that around and we have the answer to your question. Liquid oxygen would remain a non-polar molecule even while in the liquid state. Even though H2O is solid under conditions where LOX is stable, some tiny amount of H2O would still be able to dissolve in liquid oxygen for exactly the same reason; entropy. However, it wouldn’t be very much, they’d remain mostly two distinct phases, just as oxygen vapor and H2O liquid remain two distinct phases due to their chemistry.
65 notes
·
View notes
Text
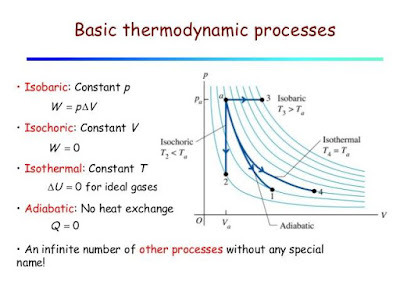
Thermodynamic Processes: Isobaric, Isochoric, Isothermal, and Adiabatic
A process in thermodynamics is the method by which the initial state of a system changes to the final state of a system. Technically speaking, there are countless types of thermodynamic processes to define each possible method in existence, most of which are unnamed. Of the specific named thermodynamic processes, there are four basic options.
Isobaric, isochoric, and isothermal (and, indeed, any other processes with the prefix iso-) are all processes in which a single variable is kept constant. For isobaric, pressure is constant, for isochoric, volume is constant, and for isothermal, temperature is constant. The fourth option, adiabatic processes, are processes in which there is no exchange of heat.
Sources/Further Reading: (Image source - Mechanics Tips blog) (University of Illinois lecture slides) (University of Central Florida) (Wikipedia) (Law of Thermodynamics Info)
51 notes
·
View notes
Text
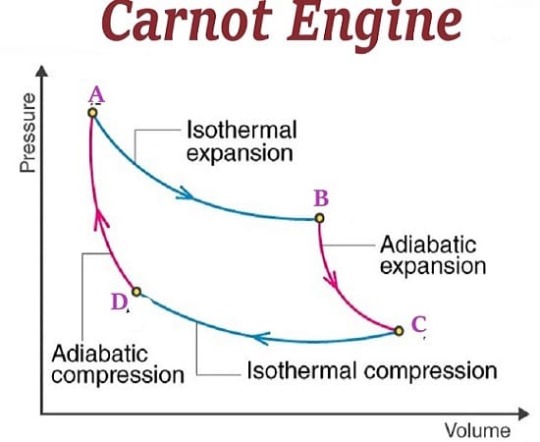
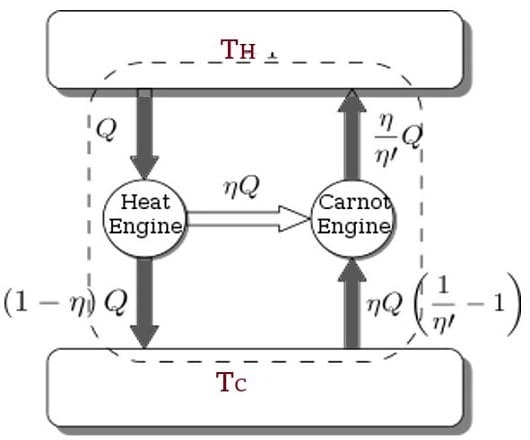
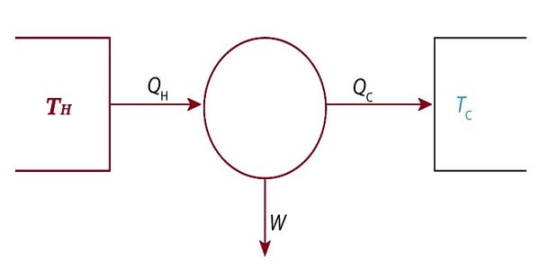
The Carnot Engine
The Carnot cycle is a specific thermodynamic cycle with four parts first designed by Nicolas Leonard Sadi Carnot. The Carnot engine, theorized by the same man, is a theoretical heat engine that operates on the Carnot cycle. It is an idealized, reversible process operating between two temperatures that represents the highest possible efficiency of a heat engine (between those two temperatures) - any irreversible process will have lower efficiency, any other reversible process will have equal efficiency. The second law of thermodynamics can be stated with the Carnot engine in mind, and mathematical study of this cycle ultimately lead to the discovery of the concept of entropy over thirty years later.
Sources/Further Reading: (Images source - Mechanical Boost) (LibreTexts) (Wikipedia)
27 notes
·
View notes
Text
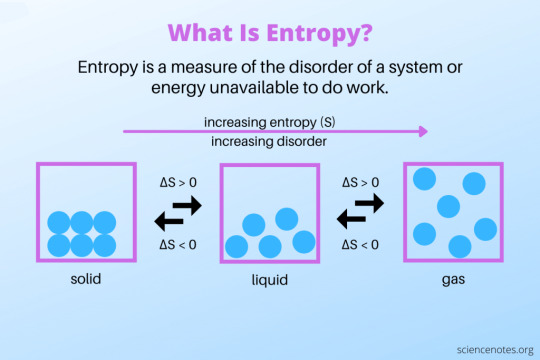
Entropy
Though the concept of entropy - or aspects of it, at least - had been discussed for some time previously, the word itself wasn't coined until 1865 by German physicist Rudolf Clausius. Clausius defined entropy as based on the state or the configuration of a system, which remains a part of the definition to this day. Entropy, simply put, is a measure of the disorder of a system. Each state of a system has a different entropy, with the more disordered the state the higher the entropy. This is an extensive property, something that depends on how much matter is in a system (volume is an extensive property, thermal conductivity is not). However, entropy cannot be directly measured and is usually calculated in terms of a change in entropy. Mathematically, entropy is typically represented by a capital S and has units of energy over temperature (in SI units, J/K).
Sources/Further Reading: (Image source - Science Notes) (Wikipedia) (RSC)
49 notes
·
View notes
Text
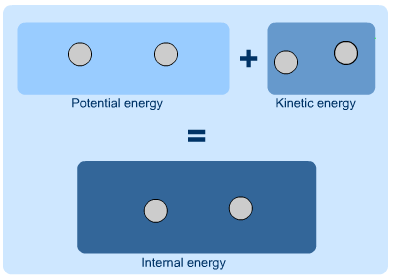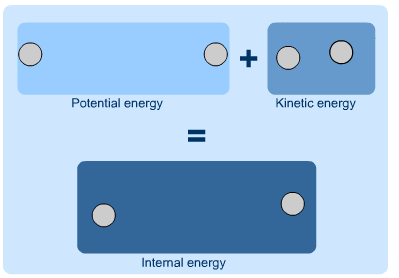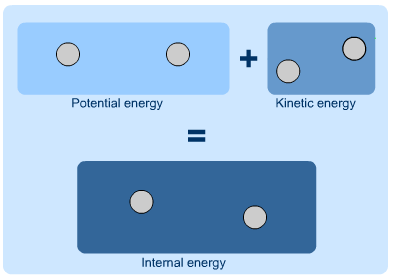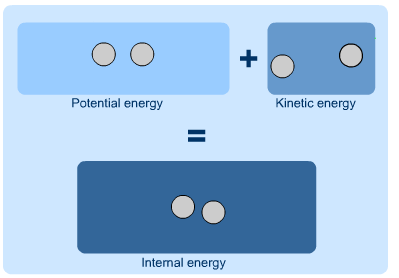
Internal Energy
As the term suggests, internal energy is the energy that is contained within a system. The potential energy of the system overall, or the kinetic energy of a moving system, are not a part of its internal energy. Instead, it is the potential and kinetic energy of the molecules and atoms of the system and make up its internal energy. If a system is isolated, the internal energy will never change. Since internal energy cannot be measured directly, it is often discussed in terms of the change in internal energy, or the energy that enters or leaves a system. Mathematically, internal energy is typically represented by a capital U, with SI units of Joules.
Sources/Further Reading: (LibreTexts) (Wikipedia) (OpenStax) (HyperPhysics)
Image source.
36 notes
·
View notes
Text
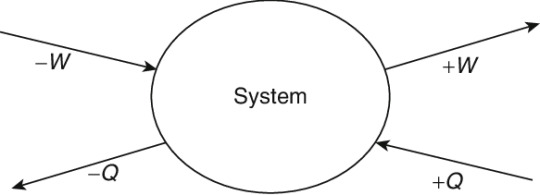
Sign Conventions in Thermodynamics
Mathematically, a negative sign has more than one meaning. Often it simply means a negative number, a value less than zero. But in many cases, the sign of a value is used to indicate direction. This is the case in thermodynamics, and, in particular, the first law of thermodynamics. If we consider a quantity of heat leaving a system, and a quantity of heat entering a system, the value of that quantity is the same - what differs is the direction the heat is moving.
Typically, heat entering a system is given a positive value, work done by a system is given a positive value, and vice versa, thereby giving us the formula that the change in internal energy is equal to heat minus work. This, however, is merely a sign convention. It would not be incorrect to give heat entering a system a negative value. The important thing with sign conventions is not the actual convention itself, but awareness of it. Using defined conventions helps eliminate confusion and errors and ensures that everyone is working off the same point of view when making calculations.
Sources/Further Reading: (Image source - book chapter) (OpenStax) (University of Wisconsin-Madison) (unacademy) (Wikipedia - Sign convention)
24 notes
·
View notes
Text
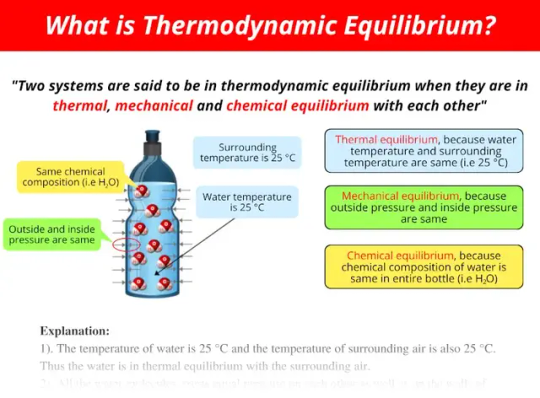
Thermodynamic Equilibrium
Equilibrium in thermodynamics occurs when there is no net flow of energy. It often refers to thermal equilibrium (the parts of the system are the same temperature), but can apply to mechanical and chemical equilibrium as well. If left undisturbed for a sufficient length of time, all systems will tend toward equilibrium. Keep in mind that this length of time can vary greatly depending on the system.
Sources/Further Reading: (Image source - laws of thermo) (NASA) (UCI lecture) (Wikipedia) (LibreTexts)
34 notes
·
View notes
Text
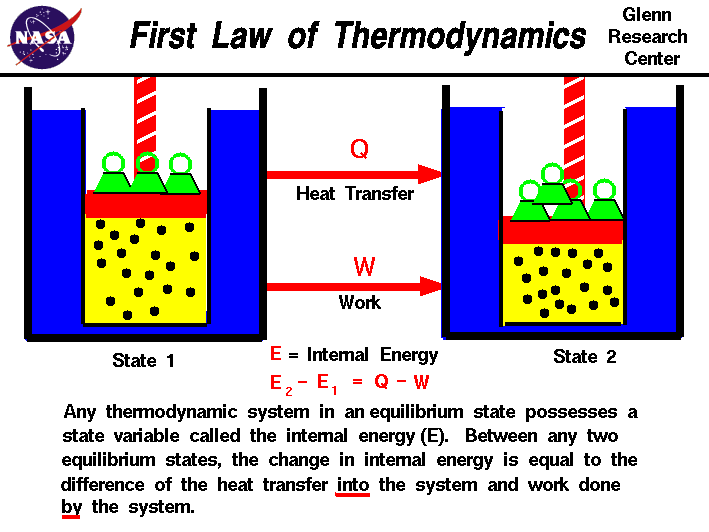
The First Law of Thermodynamics
Also known as a form of the law of conservation of energy, the first law of thermodynamics essentially states that any energy that goes into (or out of) a system alters the energy of that system by the same amount. It is written in terms of work, heat transfer, and internal energy. Mathematically, the change in internal energy of a system is equal to the difference between the heat transfer into a system and the work done by the system.
Sources/Further Reading: (Image source - NASA) (OpenStax) (Wikipedia)
37 notes
·
View notes
Text
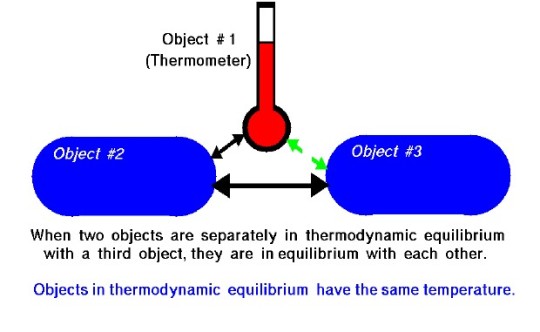
The Zeroth Law of Thermodynamics
The zeroth law of thermodynamics is one of four principal laws used to characterize and understand thermodynamic systems. It can be stated as follows: "When two objects are separately in thermodynamic equilibrium with a third object, they are in equilibrium with each other." In other words, they are said to have the same temperature. This thermodynamic law is used to define thermodynamic equilibrium and temperature.
It is called the zeroth law as the first, second, and third laws had already been established by the time it was formally stated as a law, but, as it defines equilibrium, is considered more foundational to thermodynamics (relatively) than the other laws.
Sources/Further Reading: (Image source - NASA) (MIT) (Wikipedia) (LibreTexts) (University of Utah)
33 notes
·
View notes
Text
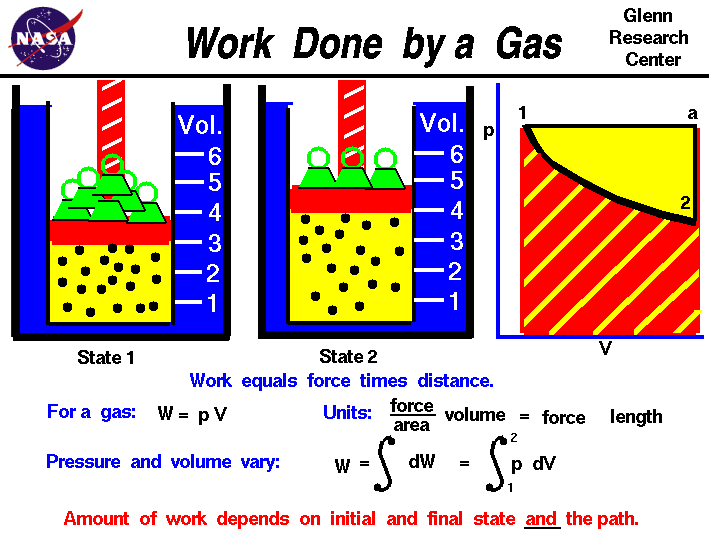
Work
In thermodynamics, work is defined as a transfer of energy - either by a system to the surroundings or vice versa. There are different forms of work, and different mathematical equations, depending on the energy in question. Pressure-volume work, where the volume and pressure of a system changes, is one of the most commonly taught forms. (The pressure-volume relationship example is typically an ideal gas in a frictionless piston, simplifying things considerably.) However, it is important to note that while work is energy, it is specifically energy in transit, though the units remain the same (J, or Joules, in SI units). In equations, work is typically represented by a capital W.
Sources/Further Reading: (Image source - NASA) (Goethe University Frankfurt) (Thermal Engineering) (LibreTexts) (Wikipedia)
20 notes
·
View notes
Text

Heat
While temperature represents the internal energy of an object or system, heat is defined as the flow of that energy. Strictly speaking, it is the transfer of thermal energy to or from a thermodynamic system (spontaneously from hot systems to cold systems). While heat is the transfer of energy, it is measured in units of energy (in SI, joules [J]), but systems cannot contain heat. In equations and formula, heat is typically represented by q or Q.
Sources/Further Reading: (Image source - Wikipedia) (The Physics Classroom) (LibreTexts) (Khan Academy) (Georgia State University)
20 notes
·
View notes
Text
Thermodynamic Temperature
Thermodynamically, temperature is defined as the measure of the average total internal energy of the system. This includes the kinetic energy of the motion of a material's atoms or molecules, among others. Thermodynamic temperature is always expressed in units of Kelvin, an absolute scale wherein 0K equates, theoretically, to zero average internal energy in a system.
Sources/Further Reading: (Image sources - NIST) (Wikipedia)
20 notes
·
View notes
Text
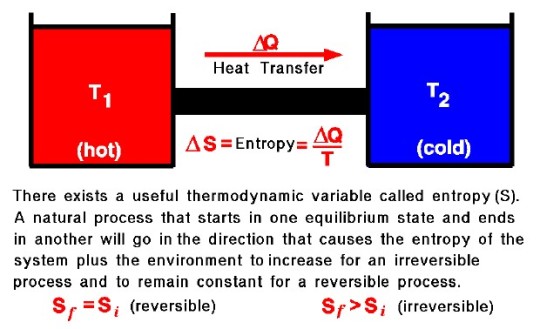
The Second Law of Thermodynamics
The second law of thermodynamics establishes the concept of entropy and constrains natural heat transfer. Simply put, it states that heat spontaneously flows from hot to cold. The second law refines scenarios that would be permissible under the first law (for example, heat flowing from cold objects to hot objects does not violate conservation of energy), narrowing down further what natural processes actually occur.
In other words, the second law could also be stated as follows:
The entropy of a cyclic process with either increase or stay the same
Not all heat can be converted to work in a cyclic process
Sources/Further Reading: (Image source - NASA) (HyperPhysics) (Wikipedia) (New Scientist)
14 notes
·
View notes
Text
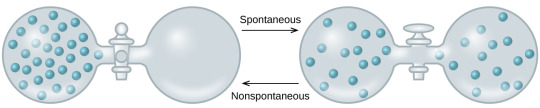

Spontaneous Processes
A spontaneous process is a process that proceeds without any external intervention or energy input. External factors, such as temperature, can influence whether a process is spontaneous in the first place, but no additional energy is added to the process. For example, above 0C/32F ice will melt spontaneously. Below that temperature it will not. (Spontaneous processes are not spontaneously reversible; spontaneous processes move toward equilibrium, not away from it.) In addition, it is important to note that speed is not a factor in determining spontaneity. One example of this is in the decay of radioisotopes, which can have wildly varying half-lives but all decay spontaneously. Mathematically, spontaneity can be determined by looking at the change in free energy of the system, or the change in entropy of both the system and the surroundings.
Sources/Further Reading: (Images source - LibreTexts) (Florida State University) (University of Massachusetts Lowell) (University of Pennsylvania) (Wikipedia)
11 notes
·
View notes