#Pharmaceutical Contract Packaging Market Industry
Text
Pharmaceutical Contract Packaging Market Growth, Overview with Detailed Analysis 2022-2028
Pharmaceutical Contract Packaging Market Growth, Overview with Detailed Analysis 2022-2028
This report studies the Pharmaceutical Contract Packaging Market with many aspects of the industry like the market size, market status, market trends and forecast, the report also provides brief information of the competitors and the specific growth opportunities with key market drivers. Find the complete Pharmaceutical Contract Packaging Market analysis segmented by companies, region, type and…
View On WordPress
#Covid-19 Impact Analysis#Pharmaceutical Contract Packaging#Pharmaceutical Contract Packaging forecast#Pharmaceutical Contract Packaging Industry#Pharmaceutical Contract Packaging Market#Pharmaceutical Contract Packaging price#Pharmaceutical Contract Packaging report#Pharmaceutical Contract Packaging research#Pharmaceutical Contract Packaging share#Pharmaceutical Contract Packaging trends
0 notes
Link

#Pharmaceutical Contract Manufacturing & Research Services#pharmaceutical industry#Pharmaceutical Contract Packaging Market#market research report#research
1 note
·
View note
Text

Saturn Formulations: Your Trusted Partner for Third Party Manufacturing Solutions
In the field of pharmaceutical Third Party Manufacturing also known as contract manufacturing offers a strategic collaboration model wherein a company outsources the production process to manufacturing Pharma products to an external entity. This enables the hiring company to access the manufacturing expertise of another firm to manufacture medication in line with stringent specifications, quality benchmark, and regulatory compliance.
Benefits of Choosing Saturn Formulations for Third Party Manufacturing in Pharma services
To compete in the pharmaceutical market, it is very important to manufacture products that are of high quality, innovative, in demand and one of the largest Pharma Companies that is offering Third Party Manufacturing is Saturn Formulations that developed a solid reputation as a third Party Pharma Manufacturer and has a solid client base in India. By associating with us you will be getting many benefits such as:
Team of Qualified professionals: - The trademark of our Third Party manufacturing company is represented by the team of professionals, doctors, experts and scientists for each and every department.
Reasonable Product price: We have high-quality affordable pharmaceutical products and the main aim of the company is to cater the needs of customers and patients.
GMP-WHO Certified Plant: Our manufacturing plant is WHO-GMP certified and that makes sure best quality products are manufactured for all our Third Party Pharma manufacturing clients.
Manufacturing Unit: This business possesses a facility capable of manufacturing goods in varying quantities, be it large-scale or small-scale. Our manufacturing unit is equipped with state-of-the art machinery, hi-tech equipment, and dedicated team having experience in the pharmaceutical industry that helps in manufacturing latest and innovative products.
Explore the Products Made by Saturn Formulations through Third Party Manufacturing
Our product range caters to a broad range of high quality products at a competitive reasonable price. All products are formulated with finest and pure raw materials and ingredients. As a premier Third Party Pharma Manufacturing company, our dedicated team ensures that our products stand out in the market for their quality. We manufactured a wide range of products under different categories to provide medical facilities to all specialised units.
Tablets
Capsules
Syrups
Dry-Syrups
Cardiac Diabetic
High-Class Injectable
Ointments, Powders, Facewash
Ayurvedic, and more.
Process of Third Party Pharma Manufacturing
Initiating order: Companies with proprietary pharmaceutical formulas and products rights commission a third Party manufacturer to manufacture a designated quantity of the product.
Quote and Contract Agreement: The manufacturer provides a cost estimate, which forms the basis for a contractual agreement outlining the work scope, encompassing production schedules, quality standards, and financial particulars.
Raw Material Provision: Third Party Pharma Manufacturing company is responsible for supplying the required raw materials and active pharmaceutical ingredients (APIs), or alternatively, the manufacturer assumes this responsibility based on the terms of the contract.
Quality products: Quality assurance measures are an important part of the production, involving batch testing, process validation, and confirming the stability of the product.
Packaging and Labelling: Following successful quality verification the product is packaged and labelled according to the need of client requirement, and in compliance with governing regulations.
Distribution: The manufactured pharmaceuticals are distributed either to the client or directly to the distributors or retail channels depending on the agreement.
Contact Details:
Name: Saturn Formulations Pvt. Ltd.
Contact Number: +91-7717389581/+91-9501686689
Email at: [email protected]
Website: www.saturnformulations.in
#pcdfranchise#pharmacompany#cardiacdiabetic#thirdpartymanufacturing#pcd pharma franchise#pcdpharmafranchise
4 notes
·
View notes
Text
Yisa Bray: What Does It Take In A Pharmacy Business?
Yisa Bray - Establishing and managing a pharmacy isn't as easy as people imagine. There are numerous things to think about. You will verify payroll, marketing, inventories, and many things besides just scripts. If you're thinking about opening a pharmacy, the following traits are crucial.
Be patient
If you lack patience, don't expect to own a drugstore. You have to wait for all the things in the pharmacy world. I like to imagine that the independent pharmacy industry is about 10 years behind every other industry in terms of speed, from payment services to government organizations to insurance contracts. It moves slowly. I'll go over the schedule for opening a pharmacy later in this article, and believe me when I say that it takes longer than you might assume or read.
Resiliency is a must.
You'll believe that no one is on your side as you cautiously begin to open your drugstore Community pharmacies aren't always profitable, therefore success takes a lot of tenacity and dedication. Some clients can take things personally, which is inappropriate in the professional world. Move on if the chance you had with the bank or the other organization didn't pan out. It is not worth the time or the effort.
Adaptability
The reason to consider this quality important for pharmacy owners is that you need to be adaptable. Most pharmacies begin as a straightforward retail pharmacies. Sometimes a retail pharmacy is insufficient. Most pharmacies end up expanding their list of pharmaceutical services to include compounding, packaging, or even specialized. These added services help keep the ship afloat in the early years and increase pharmacy revenue.
Creativity
Lastly, as per Yisa Bray, you need to be creative at all times when operating a pharmacy business. Things cannot always go the way it is planned. There are inconveniences that can be faced anytime. By being creative, you can always think of great solutions to problems. You can also put your pharmacy business ahead of others if you are creative to think of any way to make it so. Being creative can take you to further heights.
So, take these qualities by Yisa Bray along with you when you start your own pharmacy business.
12 notes
·
View notes
Text
Outsourcing for Efficiency: Exploring Contract Manufacturing in Pharmaceuticals

The world of pharmaceuticals is a complex dance between research, development, and production. While bringing a new drug to market is a triumph of science, the manufacturing side can be a logistical beast. This is where contract manufacturing steps in, offering a valuable partnership for pharmaceutical companies.
What is Contract Manufacturing?
Imagine a company with a brilliant new drug formula. They’ve poured resources into research and development, but lack the facilities or expertise for large-scale production. This is where a Contract Manufacturing Organization (CMO) comes in. A CMO is a specialized company that manufactures drugs on behalf of other businesses. They handle everything from formulation to packaging, using their expertise and facilities to bring the drug to life.
The Advantages of Outsourcing Production
There are several reasons a pharmaceutical company might choose contract manufacturing:
Cost-Effectiveness: Building and maintaining a production facility is expensive. CMOs offer a cost-effective alternative, with economies of scale and existing infrastructure.
Focus on R&D: By outsourcing production, pharmaceutical companies can free up resources to focus on what they do best: research and development of new drugs.
Expertise and Flexibility: CMOs specialize in pharmaceutical manufacturing and have the expertise to handle complex projects. They can also offer flexibility, scaling production up or down as needed.
Speed to Market: Partnering with a CMO can significantly speed up the time it takes to get a drug to market.
The Different Players: CMOs vs. CDMOs
While CMOs handle the manufacturing process, some companies offer even more comprehensive services. A Contract Development and Manufacturing Organization (CDMO) takes things a step further. They can assist with everything from pre-formulation and development to clinical trials and, of course, final production.
Ensuring Quality and Collaboration
Contract manufacturing hinges on a strong partnership. Pharmaceutical companies need to ensure CMOs meet strict quality standards and regulations. Clear communication and robust quality control procedures are essential.
The Future of Contract Manufacturing
The pharmaceutical industry is constantly evolving. As the demand for new drugs and therapies grows, contract manufacturing is likely to play an even more crucial role. CMOs will continue to develop their expertise and capabilities, offering a wider range of services and keeping pace with technological advancements.
Conclusion
Contract manufacturing is a win-win for the pharmaceutical industry. It allows drug companies to focus on innovation while leveraging the expertise of CMOs to bring life-saving treatments to patients faster and more efficiently. As the industry moves forward, contract manufacturing will undoubtedly remain a key driver of progress.
0 notes
Text
Global Top 25 Companies Accounted for 61% of total Pharmaceutical Sterile Fill-Finish market (QYResearch, 2021)
In pharmaceutical manufacturing, fill-finish operations are critical, since fill-finish is the last step before a product is packaged and ultimately delivered to the patient. By the time a drug reaches this stage, the drug product is highly valuable, as it has already been through labour- and cost-intensive production stages, including upstream processing, cell culture or fermentation and downstream purification. Failures in the integrity of the fill-finish stage can introduce microbial contamination and generate issues with formulation and dosing.
According to the new market research report “Global Pharmaceutical Sterile Fill-Finish Market Report 2023-2029”, published by QYResearch, the global Pharmaceutical Sterile Fill-Finish market size is projected to reach USD 5.15 billion by 2029, at a CAGR of 10.2% during the forecast period.
Figure. Global Pharmaceutical Sterile Fill-Finish Market Size (US$ Million), 2018-2029
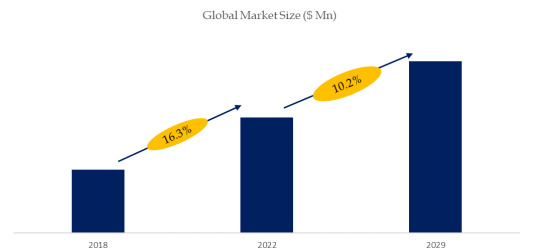
Above data is based on report from QYResearch: Global Pharmaceutical Sterile Fill-Finish Market Report 2023-2029 (published in 2023). If you need the latest data, plaese contact QYResearch..
Figure. Global Pharmaceutical Sterile Fill-Finish Top 25 Players Ranking and Market Share (Ranking is based on the revenue of 2022, continually updated)

Above data is based on report from QYResearch: Global Pharmaceutical Sterile Fill-Finish Market Report 2023-2029 (published in 2023). If you need the latest data, plaese contact QYResearch.
The global key manufacturers of Pharmaceutical Sterile Fill-Finish include Baxter BioPharma Solutions, Boehringer Ingelheim, Fresenius Kabi, Aenova, Pfizer CentreOne, Vetter Pharma, WuXi Biologics, Jubilant HollisterStier, LSNE Contract Manufacturing, Bushu Pharmaceuticals, etc. In 2022, the global top 10 players had a share approximately 61.0% in terms of revenue.
About QYResearch
QYResearch founded in California, USA in 2007.It is a leading global market research and consulting company. With over 16 years’ experience and professional research team in various cities over the world QY Research focuses on management consulting, database and seminar services, IPO consulting, industry chain research and customized research to help our clients in providing non-linear revenue model and make them successful. We are globally recognized for our expansive portfolio of services, good corporate citizenship, and our strong commitment to sustainability. Up to now, we have cooperated with more than 60,000 clients across five continents. Let’s work closely with you and build a bold and better future.
QYResearch is a world-renowned large-scale consulting company. The industry covers various high-tech industry chain market segments, spanning the semiconductor industry chain (semiconductor equipment and parts, semiconductor materials, ICs, Foundry, packaging and testing, discrete devices, sensors, optoelectronic devices), photovoltaic industry chain (equipment, cells, modules, auxiliary material brackets, inverters, power station terminals), new energy automobile industry chain (batteries and materials, auto parts, batteries, motors, electronic control, automotive semiconductors, etc.), communication industry chain (communication system equipment, terminal equipment, electronic components, RF front-end, optical modules, 4G/5G/6G, broadband, IoT, digital economy, AI), advanced materials industry Chain (metal materials, polymer materials, ceramic materials, nano materials, etc.), machinery manufacturing industry chain (CNC machine tools, construction machinery, electrical machinery, 3C automation, industrial robots, lasers, industrial control, drones), food, beverages and pharmaceuticals, medical equipment, agriculture, etc.
0 notes
Text
Sustainable Pharmaceutical Packaging Market Size Global Trends, and Opportunities Forecast by 2031
Sustainable Pharmaceutical Packaging Market SizeScope & Overview
A competitive quadrant is included in the study, which is a patented method for analyzing and evaluating a company's position based on its industry position score and market performance score. The tool divides the players into four groups based on a variety of characteristics. Financial performance during the previous years, growth plans, innovation score, new product releases, investments, market share growth, and so on are some of the elements that are examined. The study provides a thorough analysis of the worldwide Sustainable Pharmaceutical PackagingMarket Size. In-depth qualitative research, verifiable data from reliable sources, and market size predictions are all included in the report. The estimates are based on well-established research methodology.
The Sustainable Pharmaceutical Packaging market report generated using a combination of primary and secondary sources. Interviews, questionnaires, and observation of recognized industry personnel are used in the primary research. The Ansoff Matrix and Porter's 5 Forces model are used to conduct an in-depth market study in the research. In addition, the research discusses the influence of Covid-19 on the market. The report also contains information on the industry's regulatory environment, which will assist you in making an informed decision. The paper goes over the major regulatory agencies as well as the major rules and regulations that have been established on this industry in different parts of the world. The study also includes a competition analysis utilizing the analyst's competitive positioning technique, Positioning Quadrants.
Get a Sample Report https://www.snsinsider.com/sample-request/2811
Market Key Players:
Vetter Pharma International, Schott AG, Amcor plc, Gerresheimer AG, AptarGroup Inc, Owens Illinois Inc, CCL Industries Inc, SGD Pharma, West Pharmaceutical Services Inc, Drug Plastics Group, WestRock Company, Becton, Dickinson, and Company
Market Segmentation
Market segmentation by product type, application, end-user, and geography is discussed in the Sustainable Pharmaceutical Packaging research report. The research looks into the industry's growth goals, cost-cutting measures, and production procedures. A full evaluation of the core industry, including categorization and definition, as well as the structure of the supply and demand chain, is also included in the study report.
By Raw Material:
Paper & Paperboards
Plastic & Polymers
Aluminium Foil
Glass
Others
By Product type:
Primary
Secondary
Tertiary
By End users:
Contract Packaging
Institutional Pharmacy
Retail Pharmacy
Pharma Manufacturing
Competitive Outlook
The study includes a thorough examination of the market's key players, including company profiles, SWOT analyses, recent developments, and business plans. The analysis looks at all aspects of the industry, with an emphasis on major players such market leaders, followers, and newcomers. Because it clearly illustrates competitive analysis of key competitors in the Sustainable Pharmaceutical Packaging market by product, price, financial status, product portfolio, growth strategies, and geographical presence, the research is an investor's guide.
Key Objectives of Sustainable Pharmaceutical Packaging Market Report
To examine the market in terms of growth trends, prospects, and their involvement in the whole industry.
Examine competition developments such as market expansions, agreements, new product launches, and acquisitions.
Examine and research the company's market size (volume and value), key regions/countries, products, and applications, as well as background information and forecasting.
Primary global market manufacturing firms, to define, clarify, and evaluate product sales volume, value, and market share, market rivalry landscape, SWOT analysis, and future development plans.
Buy the Research Report Now https://www.snsinsider.com/checkout/2811
About Us:
SNS Insider is one of the leading Market Size research and consulting agencies that dominates the Market Size research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate Market Size data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
0 notes
Text
Biopharmaceutical CMO Market Growth Opportunities and Competitive Landscape Report to 2033
Market Definition
A Contract Manufacturing Organization (CMO), also known as a Biopharmaceutical CMO, is a company that provides manufacturing and other services to the pharmaceutical and biotechnology industries. CMOs are an important part of the pharmaceutical supply chain, and they play a vital role in bringing new drugs and therapies to market.
CMOs specialize in the manufacture of active pharmaceutical ingredients (APIs) and finished dosage forms (FDFs). They also provide a range of other services, such as analytical testing, formulation development, and packaging. CMOs are typically large, multinational companies with extensive experience in drug manufacturing.
Market Outlook
The key trends in Biopharmaceutical CMO technology are:
1. The use of biotechnology to develop new drugs and therapies.
2. The use of cell culture and fermentation technologies to produce biopharmaceuticals.
3. The use of monoclonal antibodies and other protein-based drugs.
4. The use of nucleic acid-based drugs and gene therapy.
The biopharmaceutical CMO market is driven by the increasing demand for biopharmaceuticals, the need for specialized manufacturing facilities, and the increasing number of biopharmaceutical companies. The biopharmaceutical industry is growing at a rapid pace, and the number of biopharmaceutical companies is increasing. This is resulting in an increased demand for CMOs. CMOs are specialized manufacturing facilities that are required for the production of biopharmaceuticals. They are required to meet the stringent quality standards set by the FDA. The increasing number of biopharmaceutical companies is resulting in an increased demand for CMOs.
The biopharmaceutical CMO market is facing a number of key restraints and challenges. Firstly, the market is highly competitive and there are a large number of players operating in the space. This makes it difficult for new entrants to gain a foothold in the market. Secondly, the market is capital intensive and requires significant investment in research and development. This is a major barrier for small and medium sized companies. Thirdly, the regulatory environment is constantly changing and this makes it difficult for companies to keep up with the latest regulations. Finally, the market is reliant on a small number of key customers and this makes it difficult to diversify revenue streams.
To Know More: https://www.globalinsightservices.com/reports/biopharmaceutical-cmo-market/
Research Objectives
Estimates and forecast the overall market size for the total market, across product, service type, type, end-user, and region
Detailed information and key takeaways on qualitative and quantitative trends, dynamics, business framework, competitive landscape, and company profiling
Identify factors influencing market growth and challenges, opportunities, drivers and restraints
Identify factors that could limit company participation in identified international markets to help properly calibrate market share expectations and growth rates
Trace and evaluate key development strategies like acquisitions, product launches, mergers, collaborations, business expansions, agreements, partnerships, and R&D activities
Thoroughly analyze smaller market segments strategically, focusing on their potential, individual patterns of growth, and impact on the overall market
To thoroughly outline the competitive landscape within the market, including an assessment of business and corporate strategies, aimed at monitoring and dissecting competitive advancements.
Identify the primary market participants, based on their business objectives, regional footprint, product offerings, and strategic initiatives
Request Sample: https://www.globalinsightservices.com/request-sample/GIS21593/
Market Segmentation
The biopharmaceutical CMO market report is bifurcated on the basis of product, source, service, and region. On the basis of product, it is segmented into biologics and biosimilars. Based on source, it is analyzed across mammalian and non-mammalian. By service, it is categorized into contract manufacturing, process development, packaging, and others. Region-wise, it is studied across North America, Europe, Asia-Pacific, and rest of the World.
Request Customization@ https://www.globalinsightservices.com/request-customization/GIS21593/
Major Players
The biopharmaceutical CMO market report includes players such as Toyobo Co., Ltd., Samsung Biologics, Patheon, Lonza AG, WuXi Biologics, AbbVie Inc., Binex Co., Ltd., JRS Pharma, Biomeva GmbH, and ProBioGen AG.
Request Discounted Pricing@ https://www.globalinsightservices.com/request-special-pricing/GIS21593/
Research Scope
Scope – Highlights, Trends, Insights. Attractiveness, Forecast
Market Sizing – Product Type, End User, Offering Type, Technology, Region, Country, Others
Market Dynamics – Market Segmentation, Demand and Supply, Bargaining Power of Buyers and Sellers, Drivers, Restraints, Opportunities, Threat Analysis, Impact Analysis, Porters 5 Forces, Ansoff Analysis, Supply Chain
Business Framework – Case Studies, Regulatory Landscape, Pricing, Policies and Regulations, New Product Launches. M&As, Recent Developments
Competitive Landscape – Market Share Analysis, Market Leaders, Emerging Players, Vendor Benchmarking, Developmental Strategy Benchmarking, PESTLE Analysis, Value Chain Analysis
Company Profiles – Overview, Business Segments, Business Performance, Product Offering, Key Developmental Strategies, SWOT Analysis
Buy your copy here: https://www.globalinsightservices.com/checkout/single_user/GIS21593/
With Global Insight Services, you receive:
10-year forecast to help you make strategic decisions
In-depth segmentation which can be customized as per your requirements
Free consultation with lead analyst of the report
Infographic excel data pack, easy to analyze big data
Robust and transparent research methodology
Unmatched data quality and after sales service
Contact Us:
Global Insight Services LLC
16192, Coastal Highway, Lewes DE 19958
E-mail: [email protected]
Phone: +1-833-761-1700
Website: https://www.globalinsightservices.com/
0 notes
Text
Health innovation and the future of medicines development
The report titled “Healthcare Flexible Packaging Market” has recently been added by We Market Research to get a stronger and more effective business outlook. It provides an in-depth analysis of the different attributes of the industry, such as trends, policies, and customers operating in different geographies. Research analysts use quantitative as well as qualitative analytical techniques to provide users, business owners, and industry professionals with accurate and actionable data.
The report includes an executive summary, global economic outlook, and overview sections which provide a consistent analysis of the Healthcare Flexible Packaging market. Additionally, the report in the Market Overview section outlines PLC analysis and PESTLE analysis to provide a thorough analysis of the market. The overview section details Porter's five forces analysis which helps to reveal a possible scenario of the market by disclosing a competitive scenario with respect to the Healthcare Flexible Packaging Market.
Get a Sample Copy of Report, Click Here: https://wemarketresearch.com/reports/request-free-sample-pdf/healthcare-flexible-packaging-market/1387
Key companies profiled in this research study are:
Dätwyler Holding
Inc Bemis Company
Inc Catalent Pharma Solutions
WestRock, Aptar, Inc
Mondi
Coveris S.A
Becton, Dickinson & Company
Berry Global
CCL Industries
, Winpak Ltd
Gerresheimer
Huhtamäki Oyj
Sealed Air
Healthcare Flexible Packaging Market Segmentation:
By Material
Plastics
Paper
Bioplastics
Aluminium
By Product
Seals
High Barrier Films
Wraps
Lids & Labels
Pouches & Bags
Others
By End Use
Pharmaceutical Manufacturing
Medical Device Manufacturing
Implant Manufacturing
Contract Packaging
Others
The leading players of the Healthcare Flexible Packaging industry, their market share, product portfolio, company profiles are covered in this report. Key market players are analyzed on the basis of production volume, gross margin, market value, and price structure. The competitive market scenario among Healthcare Flexible Packaging players will help the industry aspirants in planning their strategies. The statistics presented in this report are an accurate and useful guide to shaping your business growth.
This research report also presents practical and practical case studies to help you get a clearer understanding of the topic. This research report has been prepared through industry analysis techniques and presented in a professional manner by including effective information graphics whenever necessary. It helps ensure business stability and rapid development to achieve notable remarks in the global Healthcare Flexible Packaging market.
Purchase a Copy of this Healthcare Flexible Packaging Market research report at@ https://wemarketresearch.com/purchase/healthcare-flexible-packaging-market/1387?license=single
Finally, Healthcare Flexible Packaging Market report is the believable source for gaining the Market research that will exponentially accelerate your business. The report provides locales, economic conditions, item values, benefits, limits, creations, supplies, requests, market development rates, and numbers, etc. Healthcare Flexible Packaging Industry Report Announces Additional New Task SWOT Examination, Speculation Achievement Investigation and Venture Return Investigation.
Report Customization Service:
We Market Research customizes the report according to your needs. This report can be personalized to suit your requirements. Get in touch with our sales team so you can get a report tailored to your needs.
About We Market Research:
WE MARKET RESEARCH is an established market analytics and research firm with a domain experience sprawling across different industries. We have been working on multi-county market studies right from our inception. Over the time, from our existence, we have gained laurels for our deep rooted market studies and insightful analysis of different markets.
Our strategic market analysis and capability to comprehend deep cultural, conceptual and social aspects of various tangled markets has helped us make a mark for ourselves in the industry. WE MARKET RESEARCH is a frontrunner in helping numerous companies; both regional and international to successfully achieve their business goals based on our in-depth market analysis. Moreover, we are also capable of devising market strategies that ensure guaranteed customer bases for our clients.
Contact Us:
Mr. Robbin Joseph
Corporate Sales, USA
We Market Research
USA: +1-724-618-3925
Websites: https://wemarketresearch.com/
Email: [email protected]
0 notes
Text
Ensuring Industrial Efficiency with Activated Carbon Granular Manufacturers Solutions - Angel Chemicals
We are key players in the production of a versatile adsorbent material widely used in various industries, Activated Carbon Granular Manufacturers including water purification, air filtration, food and beverage processing, pharmaceuticals, and environmental remediation.

Raw Material Sourcing: It is source raw materials from various carbonaceous sources, with coconut shells and coal being among the most commonly used. The choice of raw material depends on factors such as availability, cost-effectiveness, and the desired properties . Sustainable sourcing practices, such as using renewable raw materials and ensuring ethical supply chains, are increasingly important considerations for minimize environmental impact.
Quality Control: It is critical in ensure consistency and performance of the final product. It is implement stringent quality control measures at every stage of production, including raw material testing, process monitoring, and product testing. Quality parameters such as particle size distribution, adsorption capacity, hardness, and ash content are closely monitored to meet industry standards and customer requirements.
Product Range: We are offer a diverse range of products tailored to meet the specific needs of different applications. These may include standard-grade for general-purpose water and air treatment, speciality-grade carbon for specific contaminants such as heavy metals or volatile organic compounds (VOCs), and customized products with unique properties or particle sizes. It may also offer value-added services such as impregnation with specialty chemicals or custom packaging options.
Certifications and Compliance: There are many activated carbon granular manufacturers adhere to international standards and certifications to ensure the quality and safety of their products. Common certifications include NSF/ANSI Standard 61 for drinking water treatment, ISO 9001 for quality management systems, and ASTM standards for physical and chemical properties. Compliance with regulatory requirements, such as the European Union's REACH regulation or the U.S. Food and Drug Administration's (FDA) regulations, is also essential for manufacturers serving specific markets or applications.
Market Reach: It is distribute their products through various channels, including direct sales to end-users, distributors, and retailers, as well as contracts with industrial customers and original equipment . They may also export activated carbon to international markets to meet global demand. Strong market reach and distribution networks enable to reach a wide customer base and provide timely support and services.
Research and Development: Research and development (R&D) play a crucial role in Activated Carbon Granular Suppliers to drive innovation, improve product performance, and develop new applications. It is invest in R&D initiatives to explore advanced activation techniques, optimize production processes, and enhance the sustainability. Collaboration with research institutions, universities, and industry partners helps stay at the forefront of technology and market trends.
Address : Angel Chemicals Pvt. Ltd., Plot No. 374/2, Nr. Hanuman Mandir, GIDC, Makarpura, Baroda, Gujarat - 390010
Phone No : +91-9825512916,
Email Id : [email protected]
Url : https://www.angelchemindia.com/manufacturers/activated-carbon-granular.html
#Polyelectrolyte Manufacturers#Anionic Polyelectrolyte Manufacturers#Flocculant Chemicals Manufacturers#Cationic Polyelectrolyte Manufacturers#Polyelectrolyte Powder Manufacturers#Color Removal Chemicals Manufacturers
0 notes
Text
Pharmaceutical Product Development: A Deep Dive into USSF's Expertise
In the highly competitive pharmaceutical industry, product development plays a pivotal role in bringing new and effective drugs to the market. USSF, a prominent name in the field, leverages its expertise in pharmaceutical product development to transform groundbreaking research into viable commercial products. From the meticulous planning stage to regulatory compliance and market launch, USSF's comprehensive approach ensures success at every step.
Drug Research and Development Process: The Key to Effective Medicines
Before a pharmaceutical product reaches the shelves, it undergoes an intricate and rigorous process of drug research and development (R&D). USSF's dedicated team of scientists and researchers tirelessly work to identify promising drug candidates, conduct preclinical and clinical trials, and rigorously evaluate their safety and efficacy. Through cutting-edge technology and industry best practices, USSF accelerates the R&D process, making it more efficient and cost-effective.
Contract Manufacturing of Formulations: USSF's Commitment to Quality
As a leading player in the pharmaceutical industry, USSF understands the value of contract manufacturing for formulations. Partnering with USSF for contract manufacturing allows companies to focus on their core competencies while USSF takes care of formulation development, process optimization, manufacturing, and packaging. USSF's state-of-the-art facilities and adherence to Good Manufacturing Practices (GMP) ensure that all formulations meet the highest quality standards.
USSF: Combining Precision and Innovation
USSF's success lies in its ability to strike a delicate balance between precision and innovation in every aspect of pharmaceutical product development. The company's team of scientists, engineers, and regulatory experts work collaboratively to design and optimize formulations, address challenges, and anticipate industry trends. Their forward-thinking approach allows USSF to stay ahead of the curve, constantly improving processes and delivering superior pharmaceutical products to clients and patients.
Regulatory Compliance: USSF's Cornerstone
In the pharmaceutical industry, regulatory compliance is crucial to ensure patient safety and trust. USSF's rigorous adherence to global regulatory guidelines ensures that all their processes meet the highest standards, from research and development to contract manufacturing. This commitment to safety and compliance guarantees that USSF's products are trusted by healthcare professionals and patients alike.
USSF: A Partner for Success
In the competitive landscape of pharmaceutical product development, USSF stands out as a trusted partner. With their collaborative approach, commitment to quality, and ability to navigate complex regulatory environments, USSF helps companies accelerate their drug development timelines, reduce costs, and achieve commercial success. By collaborating with USSF, organizations can leverage their vast experience and expertise to bring effective and innovative medicines to the market, making a difference in patients' lives worldwide.
In conclusion, USSF's prowess in pharmaceutical product development, their understanding of the drug research and development process, and their expertise in contract manufacturing of formulations make them an invaluable partner for companies in the pharmaceutical industry. Through their commitment to quality, compliance, and innovation, USSF continues to drive advancements in the field and deliver transformative pharmaceutical solutions.
#contract manufacturing of formulations#custom formulation manufacturing#pharmaceutical product development#cgmp vaccine adjuvant development
0 notes
Text
Clinical Trial Supplies Market to be Worth $5.59 Billion by 2031
Meticulous Research®—a leading global market research company, published a research report titled ‘Clinical Trial Supplies Market by Phase (I–IV) Service (Manufacturing, Packaging, Logistic, Documentation) Type (Biologic, Small-molecule, Medical Device) Therapy Area (Oncology, Cardiology, CNS, Immunology, Respiratory) End User - Global Forecast to 2031.’
According to this latest publication from Meticulous Research®, the global clinical trial supplies market is projected to reach $5.59 billion by 2031 at a CAGR of 7.7%. The growth of the clinical trial supplies market can be attributed to factors such as the rising number of clinical trials, the increasing decentralization of clinical trials, the proliferation of generic drugs and biopharmaceuticals, the rise in R&D expenditure among pharmaceutical and biopharmaceutical companies, and the growing need developing novel therapies. However, the high costs associated with drug development restrain the market's growth.
Furthermore, emerging economies and drug patent expirations are expected to create market growth opportunities. However, changes in the regulatory landscape and clinical trial failures, particularly in cases of rare diseases, pose significant challenges to the market's growth.
Key Players
The key players operating in the global clinical trial supplies market are Catalent, Inc. (U.S.), NUVISAN GmbH (Germany), Thermo Fisher Scientific Inc. (U.S.), Almac Group (U.K.), Eurofins Scientific SE (Luxembourg), Parexel International (MA) Corporation (U.S.), Marken (U.S.), Biocair International Limited (U.K.), KLIFO (Denmark), Piramal Pharma Limited (India), Movianto Group (U.K.), and ICON plc (Ireland).
Clinical Trial Supplies Market: Future Outlook
The global clinical trial supplies market is segmented by Clinical Phase (Phase I, Phase II, Phase III, Phase IV), Services (Manufacturing, Packaging & labeling, Logistics & Distribution, Documentation, and Other Services), Type (Medical Devices, Biologics, and Small Molecules), Therapeutic Area (Cardiology, Infectious Diseases, Oncology, CNS, Inflammation & Immunology, Metabolic Disorders, Respiratory Disorders, and Other Therapeutic Areas), End User (Pharmaceutical & Biotechnology Companies, Contract Research Organizations, Medical Device Manufacturers, and Clinical Research Organizations), and Geography. The study also evaluates industry competitors and analyzes the market at the global and regional levels.
Among all the clinical phases studied in this report, the phase III segment is expected to register the highest CAGR during the forecast period. This growth is primarily attributed to the intricacy of phase III clinical trials, characterized by large sample sizes and a high failure rate. Patient recruitment poses a significant challenge for companies conducting phase III clinical trials. Recruiting an adequate number of participants becomes a major hurdle due to the need to enroll patients who meet specific study criteria, considering both inclusions and exclusions outlined in the study design. As a result, there is a rising adoption of clinical trial supply services to address this challenge and facilitate patient recruitment. These factors contribute to the growth of this segment.
Among all the services studied in this report, in 2024, the logistics & distribution segment is expected to account for the largest share of the clinical trial supplies market. The large market share of this segment is attributed to the growing need for effectively organizing and coordinating logistics in this market, driven by the increasing volume of clinical trials conducted globally. Efficient logistics and distribution play a crucial role in mitigating the oversupply or undersupply of drugs, subsequently reducing wastage. Furthermore, the utilization of logistics and distribution services ensures enhanced transparency throughout the supply chain through real-time tracking capabilities. This transparency in supply chain operations contributes to the increased demand for logistics and distribution services in the clinical trial supplies market.
Among all the types studied in this report, the biologics segment is expected to register the highest CAGR during the forecast period. This growth can be attributed to the increasing complexity of biologics clinical trials and the low success rate of approval. The adoption of clinical trial supply services for biologics is driven by the intricacy and variability associated with these products. Biologics face a high risk of rejection during clinical trial phases due to their complexity, making it essential to ensure accurate and consistent manufacturing across all batches. For instance, the likelihood of biologics receiving approval in phase I of clinical trials is only 9.1%, while vaccines have a probability of 9.7%.
Among all the therapeutic areas studied in this report, in 2024, the oncology segment is expected to account for the largest share of the clinical trial supplies market. The large market share of this segment can be attributed to the rising incidence of cancer, the low success rate observed in clinical trials in this area, the increasing number of drugs in the clinical trial pipeline, and the increase in government funding allocated for the development of oncology drugs and clinical trials. For instance, in June 2023, the University of Birmingham (U.K). received a grant of $12 million (£10 million) from Cancer Research UK for the renewal of its Cancer Research Clinical Trials Unit. This unit facilitates more than 100 national and international trials.
Among all end users studied in this report, in 2024, the pharmaceutical & biotechnology segment is expected to account for the largest share of the clinical trial supplies market. The large market share of this segment can be attributed to the growing emphasis on personalized medicine and the rise in funding allocated for pharmaceutical research initiatives. The rising demand for new drugs and therapies is driving an increase in funding for research laboratories, thereby creating a surge in demand for clinical trial supplies. For instance, funding from the NIH for research purposes has risen significantly, increasing from $33 billion in 2015 to $42 billion in 2021 (Source: Congressional Research Service).
Geographic Review
This research report analyzes major geographies and provides a comprehensive analysis of the market in North America (U.S. and Canada), Europe (Germany, France, U.K., Italy, Spain, and the Rest of Europe), Asia-Pacific (China, Japan, India, and the Rest of Asia-Pacific), Latin America (Brazil, Mexico, and the Rest of Latin America), and the Middle East & Africa.
Among all regions studied in this report, in 2024, North America is expected to account for the largest share of the clinical trial supplies market. North America’s major market share is attributed to the presence of key market players, well-established laboratories, and substantial spending on R&D by pharmaceutical and biotechnology companies.
Download Sample Report Here @ https://www.meticulousresearch.com/download-sample-report/cp_id=5072
Key questions answered in the report-
Which are the high-growth market segments in terms of clinical phase, service, type, therapeutic area, end user, and region/country?
What was the historical market size for clinical trial supplies globally?
What are the market forecasts and estimates for the period 2024–2031?
What are the major drivers, restraints, challenges, opportunities, and trends in the global clinical trial supplies market?
Who are the major players in the global clinical trial supplies market?
What is the competitive landscape like, and who are the market leaders in the global clinical trial supplies market?
What are the recent developments in the global clinical trial supplies market?
What are the different strategies adopted by the major players in the global clinical trial supplies market?
What are the geographical trends and high growth regions/countries?
Contact Us:
Meticulous Research®
Email- [email protected]
Contact Sales- +1-646-781-8004
Connect with us on LinkedIn- https://www.linkedin.com/company/meticulous-research
0 notes
Text
The Vital Role of Pharmaceutical Contract Manufacturers

The journey of a life-saving drug from discovery to your local pharmacy shelf is a complex one. While pharmaceutical companies are household names for developing new drugs, they often rely on specialized partners to bring those drugs to fruition. This is where pharmaceutical contract manufacturers (CMOs) come in.
Who are CMOs?
CMOs are essentially manufacturing companies that specialize in producing pharmaceuticals on behalf of other companies. Think of them as the factories behind the scenes. Drug companies, often focused on research and development (RD), partner with CMOs to leverage their expertise and resources for large-scale production.
What do CMOs do?
There are two main types of CMOs:
Contract Manufacturing Organizations (CMOs): These companies handle the manufacturing of pre-formulated drugs. They receive the drug formula from the pharmaceutical company and take care of everything from sourcing raw materials to packaging the final product, ensuring strict adherence to quality and regulatory standards.
Contract Development and Manufacturing Organizations (CDMOs): These companies offer a wider range of services. They can assist with pre-formulation and formulation development, clinical trial manufacturing, and of course, large-scale commercial production.
Why are CMOs important?
CMOs play a critical role in the pharmaceutical industry for several reasons:
Reduced Costs: Building and maintaining their own manufacturing facilities can be expensive for pharmaceutical companies. Partnering with a CMO allows them to leverage existing infrastructure and expertise, leading to significant cost savings.
Faster Time to Market: CMOs have the experience and capacity to ramp up production quickly, allowing drugs to reach patients faster. This is especially crucial for life-saving medications.
Expertise and Flexibility: CMOs often specialize in specific types of drugs or dosage forms. This expertise ensures high-quality production and allows pharmaceutical companies to focus on their core competencies.
Compliance: The pharmaceutical industry is heavily regulated. CMOs maintain rigorous quality control procedures and ensure compliance with regulatory requirements like Good Manufacturing Practices (GMP).
The Future of Pharmaceutical Contract Manufacturing
The CMO industry is constantly evolving. As the demand for new and complex drugs increases, we can expect to see CMOs offering even more specialized services, such as gene therapy and biologic drug manufacturing. Additionally, with the globalized nature of the pharmaceutical industry, we can expect to see more CMOs expanding their operations internationally.
In conclusion, pharmaceutical contract manufacturers are a vital link in the drug development and production chain. By providing expertise, flexibility, and cost-effectiveness, CMOs play a critical role in bringing essential medications to patients around the world.
0 notes
Text
Pharmaceutical Contract Research and Manufacturing Market Will Hit Big Revenues In Future | Biggest Opportunity Of 2024
Pharmaceutical Contract Research and Manufacturing refers to a method in which the product's parent business does not actually create the product. Instead, the corporation decides to outsource all production processes to contract manufacturers with the expertise and infrastructure needed to produce the best version of that product at the lowest feasible cost. Contract research manufacturers, as the name implies, manufacture other company’s products on a contract basis. The parent firms then apply their own label and packaging to the items produced by contract manufacturers, and they have their own sales and distribution channels and networks to get the product to the end consumer. Contract research and manufacturing services (CRAMs) are one of the pharmaceutical and biotechnology industries' fastest growing sectors. Contract research organisations (CROs) and contract manufacturing organisations (CMOs) provide low-cost outsourcing services to the pharmaceutical industry (CMOs). Huge investments in R&D, followed by low productivity, are driving companies to reduce manufacturing costs by outsourcing research and manufacturing to low-cost countries. Outsourcing provides significant advantages over other matured pharmaceutical hubs.
Free Sample Report + All Related Graphs & Charts @: https://www.advancemarketanalytics.com/sample-report/184401-global-pharmaceutical-contract-research-and-manufacturing-market?utm_source=Organic&utm_medium=Vinay
Latest released the research study on Global Pharmaceutical Contract Research and Manufacturing Market, offers a detailed overview of the factors influencing the global business scope. Pharmaceutical Contract Research and Manufacturing Market research report shows the latest market insights, current situation analysis with upcoming trends and breakdown of the products and services. The report provides key statistics on the market status, size, share, growth factors of the Pharmaceutical Contract Research and Manufacturing The study covers emerging player’s data, including: competitive landscape, sales, revenue and global market share of top manufacturers are Patheon (Thermo Fisher Scientific) (United States), Boehringer Ingelheim (Germany), Labcorp (United States), IQVIA (United States), PPD, Inc. (United States), Sun Pharmaceutical Industries Ltd. (India), Medpace (United States), Syngene International Limited (India), WuXi AppTec (China), ICON plc (Ireland), Lonza Group AG (Switzerland),
Thanks for reading this article; you can also get individual chapter wise section or region wise report version like North America, Europe or Southeast Asia.
0 notes