#Infectious Testing Market industry
Text
Southeast Asia Molecular Diagnostics Market Outlook: Global Opportunity Analysis and Industry Forecast (2024-2031)
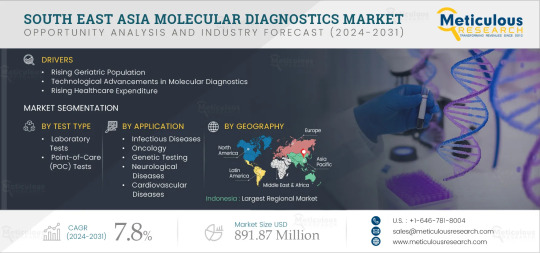
Meticulous Research®—a leading market research company, published a research report titled, ‘South East Asia Molecular Diagnostics Market by Offering (Reagents, Kits, Systems, Software), Test Type (Lab, PoC), Technology (PCR, ISH, INAAT, Sequencing, Microarray), Application (HIV, Influenza, HPV, Oncology, Gene Testing), End User - Forecast to 2031’
Download Free Sample Report@ https://www.meticulousresearch.com/download-sample-report/cp_id=5775?utm_source=pdf&utm_medium=social&utm_campaign=product&utm_content=25-04-2024
According to this latest publication from Meticulous Research®, the South East Asia molecular diagnostics market is projected to reach $891.87 million by 2031, at a CAGR of 7.8% from 2024 to 2031. The growth of the South East Asia molecular diagnostics market is driven by several factors, including the rising geriatric population, increasing prevalence of communicable & non-communicable diseases, technological advancements in molecular diagnostics, and rising healthcare expenditure. Factors such as emerging medical tourism in South East Asian countries and increasing focus on companion diagnostics are a few opportunities that would help grow the market in the future. A shortage of skilled professionals could be considered a challenge for the South East Asia molecular diagnostics market. Moreover, the lack of harmonization of the medical device regulations across South East Asian countries and the high costs of molecular diagnostic tests restrain the growth of the market.
Key Players
The key players profiled in the South East Asia molecular diagnostics market report are Sansure Biotech, Inc. (China), F. Hoffmann-La Roche Ltd. (Switzerland), Thermo Fisher Scientific Inc. (U.S.), Hologic, Inc. (U.S.), Illumina, Inc. (U.S.), Xiamen Zeesan Biotech Co., Ltd (China), QIAGEN N.V. (Netherlands), Danaher Corporation (U.S.), Abbott Laboratories (U.S), Agilent Technologies, Inc. (U.S.).
Geographic Review
The South East Asia molecular diagnostics market is segmented by Offering (Kits & Reagents, Instruments, Software & Services), Test Type (Laboratory Tests and Point-of-Care (POC) Tests), Technology (Polymerase Chain Reaction (PCR), In Situ Hybridization (ISH), Isothermal Nucleic Acid Amplification Technology (INAAT), Microarrays, Sequencing, Mass Spectrometry, and Other Technologies), Application (Infectious Diseases [Respiratory Diseases, Hepatitis, HIV, Chlamydia Trachomatis/Neisseria Gonorrhoeae, Human Papillomavirus (HPV), Healthcare-Associated Infections (HAIs), and Other Infectious Diseases], Oncology [Breast Cancer, Colorectal Cancer, Lung Cancer, Prostate Cancer, Lymphoma, Leukemia, Cervical Cancer, and Other Cancer Types], Genetic Testing, Neurological Diseases, Cardiovascular Diseases, and Other Applications), End User (Hospitals & Clinics, Diagnostic Laboratories, Academic & Research Institutes, and Other End Users). The study also evaluates industry competitors and analyzes the regional and country-level markets.
Among offerings, in 2024, the kits and reagents segment is expected to account for the largest share of the market. The large share of the segment is attributed to the commercial availability of a diverse range of diagnostic reagents & consumables, the availability of diseases-specific test kits & assays, and the growing awareness regarding early disease diagnosis.
Request Sample@ https://www.meticulousresearch.com/request-sample-report/cp_id=5775?utm_source=pdf&utm_medium=social&utm_campaign=product&utm_content=25-04-2024
Among test types, in 2024, the laboratory test segment is expected to account for the largest share of the market. The large share of the segment is attributed to the large number of laboratory tests available in hospitals and laboratories, and the high preference for laboratory tests owing to the accuracy of the test results supports the largest share of the market.
Among technologies, in 2024, the polymerase chain reaction (PCR) segment is expected to account for the largest share of the market. The large share of the segment is attributed to benefits, such as the ability to test multi-drug resistance, its use in several laboratories and clinical techniques, including DNA fingerprinting, detection of bacteria or viruses (particularly AIDS), and diagnosis of genetic disorders. Improved PCR testing capabilities in the laboratories during the COVID-19 pandemic to manage the growing burden also supported the segment’s largest share.
Among applications, in 2024, the infectious diseases segment is expected to account for the largest share of the market. The large share of the segment is attributed to the rising prevalence of infectious diseases, the increase in funding for the development of new infectious disease diagnostic tools, and the emergence of the COVID-19 pandemic. According to the World Health Organization (WHO), in 2022, the largest number of tuberculosis (TB) cases occurred in the South-East Asian region, with a prevalence of 46%. The increasing prevalence of such infections and diseases leads to the increased demand for rapid, easy-to-use, and affordable infectious disease testing tools, therefore driving the growth of the segment.
Among end users, the hospitals & clinics segment is expected to account for the largest share of the market in 2024. The large share of the segment is attributed to the increased number of hospitalizations due to various diseases requiring molecular diagnosis and the proliferation of hospitals and clinics in emerging countries, leading to the growth in the utilization of molecular diagnostic products. The emergence of new hospitals in South East Asian countries is leading to the growing demand for molecular diagnostic tests. For instance, in March 2023, Adventist Hospital was launched in Palangka Raya, Central Kalimantan, Indonesia, which is a 51-bed, state-of-the-art hospital that offers general medicine, surgery, obstetrics and gynecology, pediatrics, and critical care services to the inhabitants of Palangka Raya and nearby communities. This not only increases the demand for molecular tests but also provides these advanced technologies to people living in rural areas.
Among geographies, in 2024, Indonesia is expected to account for the largest share of the market. The large share of the segment is attributed to the increasing prevalence of communicable & non-communicable diseases, growing adoption of molecular diagnostics, increasing prevalence of cancer and musculoskeletal diseases, rising disposable income, improvements in the healthcare infrastructure, and the growing healthcare expenditure. The increasing prevalence of diseases is driving the need for better healthcare options among patients. In such cases, increasing healthcare expenditure can help increase people’s access to healthcare and improve the quality of care. For instance, the healthcare expenditure in Indonesia increased from USD 8.07 billion in 2019 to USD 17.62 billion in 2022.
Buy Now@ https://www.meticulousresearch.com/Checkout/49985136?utm_source=pdf&utm_medium=social&utm_campaign=product&utm_content=25-04-2024
About Meticulous Research®:
Meticulous Research® is a leading global market research company that helps businesses make better decisions through insightful market intelligence and comprehensive industry analysis. With a repository of over [Number] reports spanning various industries, Meticulous Research® offers unparalleled expertise and actionable insights to empower organizations to navigate complex market challenges and achieve sustainable growth.
Contact Us:
Meticulous Research®
Email- [email protected]
Contact Sales- +1-646-781-8004
Connect with us on LinkedIn- https://www.linkedin.com/company/meticulous-research
0 notes
Text
IVD Contract Manufacturing Services Market - Global Opportunity Analysis and Industry Forecast (2024-2031)
Meticulous Research®—a leading market research company, published a research report titled, 'IVD Contract Manufacturing Services Market by Type (Assay Development, Manufacturing), Category (Reagents, Systems), Technology (Immunoassay, Molecular Diagnostics, Clinical Chemistry, Hematology, Microbiology, Urinalysis) – Global Forecast to 2031’
According to this latest publication from Meticulous Research®, the IVD contract manufacturing services market is projected to reach $25.80 billion by 2030, at a CAGR of 7.8% from 2024 to 2031. The growth of the IVD contract manufacturing services market is driven by several factors, including the rising prevalence of infectious diseases, the shift in focus from centralized laboratories to point-of-care testing services, regulatory complexities faced by IVD companies, and the need for cost-effective manufacturing of IVD tests. Factors such as high economic growth and increased outsourcing to emerging countries serve as an opportunity that would help grow the market in the future.
Factors such as the lack of skilled professionals could be considered as a challenge for the IVD contract manufacturing services market. However, maintaining product quality and protection of proprietary information restrains the growth of the market.
Key Players
The key players profiled in the IVD contract manufacturing services market report are Cenogenics Corporation (U.S.), In-Vitro Diagnostic Developers, Inc. (IDxDI) (U.S.), Savyon Diagnostics (Israel), KMS Systems, Inc. (U.S.), Nova Biomedical (U.S.), LRE Medical (Germany), Cone Bioproducts (U.S.), Invetech, Inc. (Australia), Avioq, Inc. (U.S.), TCS Biosciences Ltd. (U.K.), Affinity Life Sciences, Inc. (U.S.), Coris BioConcept (Belgium), Meridian Bioscience, Inc. (U.S.), Affinity Biologicals, Inc. (Canada), Biokit S.A. (Spain), Merck KgaA (Germany), Thermo Fisher Scientific, Inc. (U.S.), and Maxim Biomedical, Inc. (U.S.).
The IVD contract manufacturing services market is segmented by Type (Assay Development Services, Manufacturing Services, and Other Services), Category (Reagents & Consumables and Instruments & Systems), Technology (Immunoassay, Molecular Diagnostics, Clinical Chemistry, Hematology, Microbiology, and Urinalysis), and Geography. The study also evaluates industry competitors and analyzes the regional and country-level markets.
Based on type, in 2024, the manufacturing services segment is expected to account for the largest share of the market. Manufacturing services are services that include ISO-certified manufacturing facilities that allow them to carry out full project and production management, quality and regulatory support, and flexibility in scale and process, which results in increased demand for manufacturing services.
Based on category, in 2024, the reagents & consumables segment is expected to account for the largest share of the market. Reagents & consumables are products that are used for the in-vitro examination of specimens derived from the human body during disease diagnosis, treatment monitoring, prognosis, and evaluation of the patient’s health status. The large market share of the segment is attributed to factors such as increasing virulence of infectious diseases, growth of companion diagnostics, and rapid growth in molecular testing, which leads to an increased demand for POC diagnostic kits, therefore increasing the use of reagents & consumables used in the tests.
Based on technology, in 2024, the immunoassay segment is expected to account for the largest share of the market. The large share of the segment is attributed to factors such as a higher preference for immunodiagnostics owing to its inherent specificity, high throughput, and the emergence of advanced diagnostic immunoassay formats. Further, the increasing use of immunoassays in Point of Care (PoC) infectious disease testing, the need for developing novel tests, increasing usage of miniaturized devices, rising demand attributed to an aging population, and the rising trend of automation in immunoassay instruments are driving the growth of this segment.
This research report analyzes major geographies and provides a comprehensive analysis of North America (U.S., Canada), Europe (Germany, France, U.K., Italy, Spain, and the Rest of Europe), Asia-Pacific (China, Japan, India, and Rest of Asia-Pacific), Latin America and the Middle East & Africa. In 2024, North America is expected to account for the largest share of the IVD contract manufacturing services market, followed by Europe and Asia-Pacific. North America’s major market share is attributed to the rising prevalence of various infectious diseases, the growing healthcare sector, increasing awareness regarding early disease diagnosis, growing adoption of advanced innovative products, and increasing funding activities coupled with novel advanced diagnostic technologies.
Download Sample Report Here @ https://www.meticulousresearch.com/download-sample-report/cp_id=5226
Key questions answered in the report:
Which are the high-growth market segments in terms of IVD contract manufacturing services type, category, technology, and geography?
What was the historical market for IVD contract manufacturing services across the globe?
What are the market forecasts and estimates for the period 2024–2031?
What are the major drivers, restraints, opportunities, and challenges in the IVD contract manufacturing services market?
Who are the major players in the IVD contract manufacturing services market?
What is the competitive landscape, and who are the market leaders in the IVD contract manufacturing services market?
What are the recent developments in the global IVD contract manufacturing services market?
What are the different strategies adopted by the major players in the global IVD contract manufacturing services market?
What are the geographical trends and high-growth regions/countries?
Contact Us:
Meticulous Research®
Email- [email protected]
Contact Sales- +1-646-781-8004
Connect with us on LinkedIn- https://www.linkedin.com/company/meticulous-research
#IVD Contract Manufacturing Services Market#Contract Manufacturing Services#IVD Manufacturing#In-vitro Diagnostics Contract Manufacturing Services#Immunoassays#Assay Development
0 notes
Text
Informative Report on Clinical Biomarker Market | BIS Research

Clinical Biomarkers are measurable indicators of biological processes, conditions, or states within an organism. These can include molecules, genes, proteins, cells, or other substances that are detected and measured to assess normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention
The global clinical biomarkers market was valued at $24.80 billion in 2023 and is expected to reach $53.20 billion by 2033, growing at a CAGR of 7.93% between 2023 and 2033.
Clinical Biomarker Overview
Clinical Biomarkers are measurable indicators, such as molecules, genes, or proteins, that signal biological processes, disease states, or responses to treatment within an organism.
The era of Clinical Biomarker market heralds a paradigm shift in healthcare, wherein treatments are tailored to individual patients based on their unique genetic makeup, biomarker profiles, and clinical
characteristics.
Role of Clinical Biomarkers
Prognosis and Disease Progression
In addition to diagnosis, biomarkers play a pivotal role in assessing disease severity, progression, and prognosis. By monitoring changes in biomarker levels over time, healthcare providers can tailor treatment plans and predict patient outcomes with greater accuracy.
Therapeutic Monitoring:
Biomarkers also serve as markers of treatment efficacy and safety. In fields such as oncology, for instance, tumor biomarkers can gauge the response to chemotherapy or targeted therapies, guiding adjustments to treatment regimens as needed
Download the report and get to know the interesting facts Click Here !
Market Dynamics
Market Drivers
Growing Demand for Clinical Biomarker Products
Increase in Industrial Activity in Clinical Biomarker Landscape
Environment Changes Provoking Swift Care and Diagnosis
Market Restraints
High Price of Products/Services Limiting Adoption of Clinical Biomarkers in Low-Income Countries
Complex Regulatory Frameworks Delaying Approval of New Clinical Biomarkers Tests
Discovering New Biomarkers Presents Difficulty
Market Opportunities
Technological Advancement in Biomarker Testing
Increased Research Funding for Executing Research and Development Exercise
Discovery of Novel Biomarkers Expanding Precision Medicine Horizons
Grab a look at our sample page click here!
Market Segmentation
By Product Type
By Clinical Area
By Technology
By End Users
Click here to visit our Precision medicine page !
China has been able to procure its place as one of the leading contributors to the clinical diagnostics market in the past five years. Major growth was significantly attributed to the increasing adoption of clinical biomarkers in oncology or rare disease space.
Uses of Clinical Biomarkers
Disease Diagnosis and Prognosis
Drug Development and Clinical Trials
Personalized Medicine
Prognosis Assessment
Cancer Detection and Monitoring
Cardiovascular Risk Assessment:
Infectious Diseases
Environmental and Occupational Exposure Monitoring
Key Players in Clinical Biomarker Market
Abbott Laboratories
Agilent Technologies, Inc.
ALCEN
Recent Developments in the Global Clinical Biomarkers Market
•In August 2023, Quest Diagnostics launched the AD-Detect test for Alzheimer’s disease in the U.S., offering consumers the first opportunity to acquire and evaluate a blood-based biomarker test for assessing the potential risks of developing AD
•In September 2023, Becton, Dickinson and Company partnered with Navigate BioPharma Services, Inc. to develop and commercialize flow cytometry-based companion diagnostics and clinical decision tools. The collaboration combined Navigate BioPharma's expertise in biomarker assay design for clinical trials with BD's extensive portfolio of flow cytometry instruments, reagents, software, and in vitro diagnostics (IVD) development services.
Key Question Answers
QWhat are the major market drivers, challenges, and opportunities in the global clinical biomarkers market?
Q What are the business development strategies, such as business expansion, acquisitions, and funding, which are implemented by the major players to sustain in the competitive market?
Q Which is the dominant product and service type developed by the leading and emerging players for clinical biomarkers?
QHow is each segment of the market expected to grow during the forecast period from 2023 to 2033?
Conclusion
Biomarkers play a pivotal role in disease diagnosis, prognosis assessment, drug development, clinical trials, and personalized treatment strategies. Their utility extends to diverse areas, including oncology, cardiology, neurology, infectious diseases, and environmental health.
The clinical biomarkers market is witnessing significant growth and innovation, driven by the increasing demand for personalized medicine, advancements in diagnostic technologies, and the expanding scope of applications across healthcare and research domains
0 notes
Text
Laboratory Microplates, Global Market Size Forecast, Top 10 Players Rank and Market Share
Laboratory Microplates Market Summary
According to the new market research report “Global Laboratory Microplates Market Report 2023-2029”, published by QYResearch, the global Laboratory Microplates market size is projected to reach USD 139.4 million by 2029, at a CAGR of 5.9% during the forecast period.

Figure. Global Laboratory Microplates Market Size (US$ Million), 2018-2029

Based on or includes research from QYResearch: Global Laboratory Microplates Market Report 2023-2029.
Market Drivers:
Growth in Life Sciences and Medical Diagnostics: The rapidly advancing biotechnology and medical fields require high-throughput experiments and efficient sample processing, which has contributed to the growing demand for Laboratory Microplatess.
Drug Development and Screening: The pharmaceutical industry requires large-scale drug screening and pharmacodynamic studies, and Laboratory Microplatess provide an efficient method to test multiple compounds or samples simultaneously.
Vaccines and clinical trials: Vaccine development and clinical trials require testing of large numbers of samples, and Laboratory Microplatess help scale up sample testing.
Laboratory Automation: Laboratory Microplatess can be integrated with automated laboratory equipment to increase experimental efficiency and precision and reduce human error.
Rapid diagnosis and biomarker research: Laboratory Microplatess can be used to detect biomarkers, which is helpful in cancer diagnosis, infectious disease detection and other fields.
Restraint:
Cost: High-quality Laboratory Microplatess are expensive to manufacture, which may limit adoption by some laboratories and research institutions.
Standardization issues: The lack of unified Laboratory Microplates standards may lead to incompatible products from different suppliers.
Complex sample handling: Complex sample preparation and handling can lead to experimental instability and reproducibility issues.
Opportunity:
Personalized Medicine: With the rise of personalized medicine, Laboratory Microplatess can be used to analyze samples from individual patients to guide the development of personalized treatment plans.
Nanotechnology and microfluidic technology: Laboratory Microplatess combine nanotechnology and microfluidic technology to achieve higher sensitivity, smaller sample volumes, and faster experimental speeds, expanding application fields.
Green Technologies: The development of alternatives to organic solvents and the rise of green laboratories may impact Laboratory Microplates materials and manufacturing processes to reduce environmental impact.
Data Analytics and Artificial Intelligence: The development of data analytics and artificial intelligence can improve the interpretation and utilization of results from Laboratory Microplates experiments, accelerating the discovery and application process.
Figure. Global Laboratory Microplates Top 10 Players Ranking and Market Share(Continually updated)
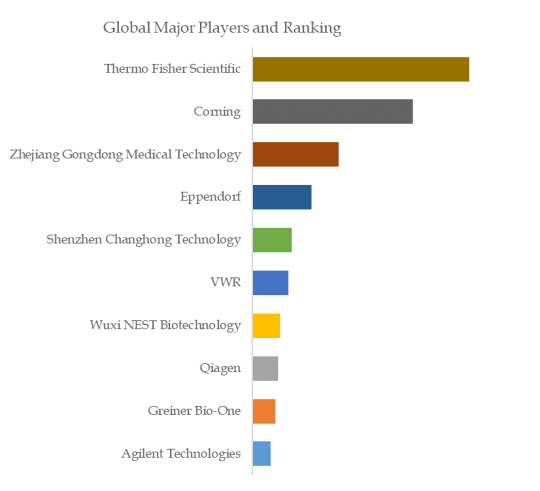
Based on or includes research from QYResearch: Global Laboratory Microplates Market Report 2023-2029.
Globally, major Laboratory Microplates manufacturers include Agilent Technologies, Greiner Bio-One, Qiagen, etc., among which the top five manufacturers account for approximately xx market share.
Figure. Laboratory Microplates, Global Market Size, Split by Product Segment

Based on or includes research from QYResearch: Global Laboratory Microplates Market Report 2023-2029.
In terms of product type, 96-Wellis the largest segment.
Figure. Laboratory Microplates, Global Market Size, Split by Application Segment

Based on or includes research from QYResearch: Global Laboratory Microplates Market Report 2023-2029.
In terms of product application, Pharmaceutical Companies is the largest application.
Figure. Laboratory Microplates, Global Market Size, Split by Region (Production)


Based on or includes research from QYResearch: Global Laboratory Microplates Market Report 2023-2029.
Figure. Laboratory Microplates, Global Market Size, Split by Region

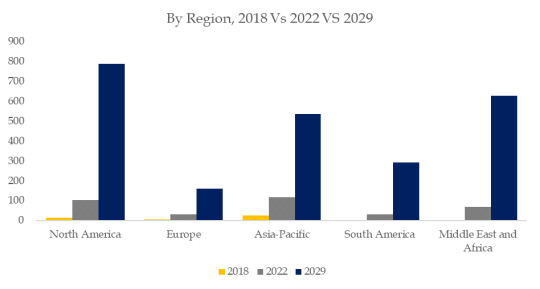
Based on or includes research from QYResearch: Global Laboratory Microplates Market Report 2023-2029.
About QYResearch
QYResearch founded in California, USA in 2007.It is a leading global market research and consulting company. With over 16 years’ experience and professional research team in various cities over the world QY Research focuses on management consulting, database and seminar services, IPO consulting, industry chain research and customized research to help our clients in providing non-linear revenue model and make them successful. We are globally recognized for our expansive portfolio of services, good corporate citizenship, and our strong commitment to sustainability. Up to now, we have cooperated with more than 60,000 clients across five continents. Let’s work closely with you and build a bold and better future.
QYResearch is a world-renowned large-scale consulting company. The industry covers various high-tech industry chain market segments, spanning the semiconductor industry chain (semiconductor equipment and parts, semiconductor materials, ICs, Foundry, packaging and testing, discrete devices, sensors, optoelectronic devices), photovoltaic industry chain (equipment, cells, modules, auxiliary material brackets, inverters, power station terminals), new energy automobile industry chain (batteries and materials, auto parts, batteries, motors, electronic control, automotive semiconductors, etc.), communication industry chain (communication system equipment, terminal equipment, electronic components, RF front-end, optical modules, 4G/5G/6G, broadband, IoT, digital economy, AI), advanced materials industry Chain (metal materials, polymer materials, ceramic materials, nano materials, etc.), machinery manufacturing industry chain (CNC machine tools, construction machinery, electrical machinery, 3C automation, industrial robots, lasers, industrial control, drones), food, beverages and pharmaceuticals, medical equipment, agriculture, etc.
0 notes
Text
Medical Swabs Market Future Scope Demands and Projected Industry Growths to 2031

The global medical swabs market has been experiencing significant growth over the past few years and is projected to continue its upward trajectory in the coming decade. According to recent market research, the market size was valued at USD 2.95 billion in 2022 and is expected to reach USD 5.01 billion by 2030, with a compound annual growth rate (CAGR) of 6.8% during the forecast period of 2023-2030. This remarkable growth can be attributed to various factors driving demand, along with emerging trends and opportunities reshaping the landscape of the medical swabs industry.
Emerging Trends and Opportunities:
Rising Demand for Point-of-Care Testing (POCT): With the increasing emphasis on early disease detection and rapid diagnosis, there has been a growing demand for point-of-care testing. Medical swabs play a crucial role in sample collection for various POCT applications, including infectious disease screening, genetic testing, and drug testing.
Advancements in Material and Design: Manufacturers are focusing on developing innovative swab designs and materials to enhance sample collection efficiency and patient comfort. The emergence of materials like flocked nylon and rayon, along with advanced designs such as foam-tipped swabs, is driving market growth.
Expanding Applications in Forensic and Veterinary Sciences: Medical swabs are not only used in clinical settings but also find applications in forensic investigations and veterinary diagnostics. The increasing adoption of DNA collection swabs in forensic analysis and sample collection in veterinary medicine is opening up new avenues for market players.
Download Free Sample Report: https://www.snsinsider.com/sample-request/2979
Key Drivers Propelling Growth:
Growing Emphasis on Infection Control: With the rise in healthcare-associated infections (HAIs) and the ongoing COVID-19 pandemic, there has been a heightened focus on infection control measures. Medical swabs are essential for specimen collection, environmental surface sampling, and wound care, driving their demand in healthcare facilities worldwide.
Increasing Number of Surgical Procedures: The rising prevalence of chronic diseases, coupled with an aging population, is leading to a surge in surgical procedures globally. Medical swabs are extensively used in surgical settings for preoperative skin preparation, wound cleaning, and specimen collection, fueling market growth.
Technological Advancements in Healthcare Infrastructure: The integration of advanced technologies such as robotics, automation, and artificial intelligence (AI) in healthcare infrastructure is streamlining sample collection processes and improving diagnostic accuracy. This technological evolution is boosting the demand for specialized medical swabs tailored for automated systems.
Challenges and Considerations:
Supply Chain Disruptions: The medical swabs market is susceptible to supply chain disruptions, especially during global health crises or natural disasters. Ensuring a resilient and diversified supply chain is crucial for mitigating risks and maintaining uninterrupted product availability.
Stringent Regulatory Compliance: Compliance with stringent regulatory requirements, including quality standards and product certifications, poses a challenge for market players. Adhering to regulatory frameworks while innovating and introducing new products requires substantial investments in research and development.
Price Sensitivity in Developing Regions: In developing regions, price sensitivity among healthcare providers and budget constraints in public healthcare systems may hinder market growth. Manufacturers need to adopt pricing strategies that accommodate diverse market segments without compromising on product quality.
Key Takeaways from the Market:
The medical swabs market is poised for substantial growth, driven by factors such as the increasing prevalence of infectious diseases, technological advancements, and the expanding applications beyond clinical settings.
Market players should focus on product innovation, strategic collaborations, and geographic expansion to capitalize on emerging opportunities and gain a competitive edge.
Addressing challenges related to supply chain resilience, regulatory compliance, and pricing strategies will be imperative for sustained growth and market penetration, particularly in diverse global markets.
In conclusion, the medical swabs market presents lucrative opportunities for manufacturers and stakeholders, fueled by evolving healthcare needs and technological advancements. By navigating the emerging trends, addressing key drivers, and overcoming challenges, players can unlock the full potential of this dynamic market and contribute to improving healthcare outcomes worldwide.
0 notes
Text
Microbial Identification Market Projected to be Resilient During 2024-2034
Market Definition
Microbial Identification is used to protect against the intentional interference of electronic signals. This equipment can be used to protect against radio frequency interference (RFI), electromagnetic interference (EMI), or other types of signal interference. There are a variety of anti-jamming technologies that can be used, depending on the type of interference that is being protected against. For example, RFI jamming can be countered with frequency-hopping spread spectrum (FHSS) technology, while EMI jamming can be countered with pulse-code modulation (PCM) or other digital modulation techniques.
Market Outlook
The global Microbial Identification Market was valued at USD 4.1 Billion in 2022 and it is anticipated to grow up to USD 13.3 Billion by 2032, at a CAGR of 12.5% during the forecast period.
The rising prevalence of infectious diseases caused by microorganisms is increasing the need for microbial identification tests across the globe. The classification of microorganisms and microbes plays a vital role in disease analysis and providing appropriate treatment. In addition, the study of bacteria, parasites, fungi, and microbes is encouraging investors in the pharmaceutical industry to finance innovation in drug development. This represents another factor positively influencing the market. Apart from this, the increasing food safety concerns around the world are driving the need for microbial identification techniques to identify food spoilage contaminants. These techniques help retain the authenticity and flavor of processed food products and increase their shelf life. Furthermore, continuous advancements in the field of microbiology and microbial identification equipment are creating a positive market outlook. Moreover, various initiatives undertaken by governments of various countries to prevent the spread of infectious diseases, coupled with the increasing expenditure on the healthcare industry, are anticipated to propel the market growth.
However, the high cost of automated microbial identification systems is likely to impede the market growth over the forecast period. Also, the high cost of automated microbial identification systems is likely to impede the market growth over the forecast period.
To Know More: https://www.globalinsightservices.com/reports/microbial-identification-market/
Research Objectives
Estimates and forecast the overall market size for the total market, across product, service type, type, end-user, and region
Detailed information and key takeaways on qualitative and quantitative trends, dynamics, business framework, competitive landscape, and company profiling
Identify factors influencing market growth and challenges, opportunities, drivers and restraints
Identify factors that could limit company participation in identified international markets to help properly calibrate market share expectations and growth rates
Trace and evaluate key development strategies like acquisitions, product launches, mergers, collaborations, business expansions, agreements, partnerships, and R&D activities
Thoroughly analyze smaller market segments strategically, focusing on their potential, individual patterns of growth, and impact on the overall market
To thoroughly outline the competitive landscape within the market, including an assessment of business and corporate strategies, aimed at monitoring and dissecting competitive advancements.
Identify the primary market participants, based on their business objectives, regional footprint, product offerings, and strategic initiatives
Request Sample: https://www.globalinsightservices.com/request-sample/GIS10521/
Market Segmentation
The report analyses the global Microbial Identification Market based on technology, application, end-user, and region.
Global Microbial Identification Market by Product & Service
On the basis of Product & Service, it is segmented into Instruments, Consumables, and Services. The instruments segments are also reported to show a constant growth rate over the forecast period owing to the rising awareness among the general population about healthcare and the increasing rate of surgical procedures. For instance, in September 2019, Guthrie, a U.S.-based integrated health care system, deployed Wolters Kluwer’s POC Advisor for the detection and treatment of sepsis at four area hospitals.
Global Microbial Identification Market by Method
By Method, the industry is classified into Phenotypic Methods, Genotypic Methods, Proteomics-based Methods. Among these Phenotypic Methods segment accounted for USD xx Million in 2021. Phenotypic Methods of Classifying and Identifying Microorganisms. Classification seeks to describe the diversity of bacterial species by naming and grouping organisms based on similarities. Phenotypic methods offer an advantage over genotypic methods in that they can identify a wide range of taxa, detect the resistance currently expressed, and resist genetic variability in resistance detection. Conventional phenotypic identification may involve a number of methods, including observation of growth and colony morphology on various media, analysis of manual biochemical reactions, and the use of automated and nonautomated commercially available biochemical panels.
Global Microbial Identification Market by Application
By application, microbial identification market has been segmented into diagnostics, food and beverage testing, pharmaceuticals, cosmetics and personal care products testing, others. During the forecast period, the food and beverage testing segment is expected to grow at the fastest CAGR of xx% in the Microbial Identification Market. Accurate microbiological testing technology is vital for the detection of foodborne pathogens, possible food spoilage organisms and technological strains. Rapid identification/confirmation and strain typing methods enable food and beverage manufacturers to make fast quality and safety decisions.
Region-wise, it is studied across North America, Europe, Asia Pacific, and the Rest of the World. North America is expected to dominate the microbial identification market over the forecast period owing to the factors such as the rising burden of infectious diseases and outbreaks of epidemics, growing healthcare expenditure, and the presence of well-established healthcare infrastructure. The increasing prevalence of infectious diseases in the region is the key factor fuelling the market growth. For instance, As per the data published by the Centers for Medicare & Medicaid Services, in March 2022, titled “CMS Office of the Actuary Releases 2021-2030 Projections of National Health Expenditures”, it has been observed that the annual growth in national health spending is expected to be average 5.1% over 2021-2030. Also, the National Health Spending in 2020 was USD 4.1 trillion and it is projected to reach USD 6.8 trillion by 2030. Thus, the increasing healthcare spending is expected to increase company activities and government initiatives in developing technologically advanced testing products and equipment, thereby propelling the market growth.
In addition, rising technologically advanced products and the presence of key market players in the region are also expected to boost market growth over the forecast period. For instance, in January 2022, BD received 510(k) clearance from the United States Food and Drug Administration for BD Kiestra IdentifA system which is designed to automate the preparation of microbiology bacterial identification testing. Also, in January 2021, Bruker Corporation launched MBT Sepsityper Kit US IVD for rapid microbial identification of more than 425 microorganisms from positive blood cultures on the MALDI Biotyper CA System. Thus, owing to the aforementioned factors, the studied market is expected to grow over the forecast period..
Request Customization@ https://www.globalinsightservices.com/request-customization/GIS10521/
Major Players
The key players in the Microbial Identification Market are as Beckman Coulter Inc. (Danaher Corporation), Biolog Inc.,, BioMerieux SA,, Bruker Corporation, Charles River Laboratories International Inc,, Eurofins Scientific SE, Liofilchem S.r.l, Merck KGaA, Shimadzu Corporation, Thermo Fisher Scientific Inc., VWR International LLC., Wickham Micro Limited. among others.
Request Discounted Pricing@ https://www.globalinsightservices.com/request-special-pricing/GIS10521/
Research Scope
Scope – Highlights, Trends, Insights. Attractiveness, Forecast
Market Sizing – Product Type, End User, Offering Type, Technology, Region, Country, Others
Market Dynamics – Market Segmentation, Demand and Supply, Bargaining Power of Buyers and Sellers, Drivers, Restraints, Opportunities, Threat Analysis, Impact Analysis, Porters 5 Forces, Ansoff Analysis, Supply Chain
Business Framework – Case Studies, Regulatory Landscape, Pricing, Policies and Regulations, New Product Launches. M&As, Recent Developments
Competitive Landscape – Market Share Analysis, Market Leaders, Emerging Players, Vendor Benchmarking, Developmental Strategy Benchmarking, PESTLE Analysis, Value Chain Analysis
Company Profiles – Overview, Business Segments, Business Performance, Product Offering, Key Developmental Strategies, SWOT Analysis
Buy your copy here: https://www.globalinsightservices.com/checkout/single_user/GIS10521//
With Global Insight Services, you receive:
10-year forecast to help you make strategic decisions
In-depth segmentation which can be customized as per your requirements
Free consultation with lead analyst of the report
Infographic excel data pack, easy to analyze big data
Robust and transparent research methodology
Unmatched data quality and after sales service
Contact Us:
Global Insight Services LLC
16192, Coastal Highway, Lewes DE 19958
E-mail: [email protected]
Phone: +1-833-761-1700
Website: https://www.globalinsightservices.com/
0 notes
Text
Diagnostic Lab Industry Size & Share, Trends Outlook (2024-2029)
The diagnostic market in India is a vital component of the healthcare ecosystem, facilitating disease detection, management, and prevention. This article delves into the dynamic landscape of diagnostic labs, exploring key factors driving growth, major players shaping the industry, emerging trends, and regional market analysis.
Analysis Projection and Diagnostic Lab Market

Diagnostic Lab Market is experiencing significant growth, with projections indicating substantial expansion in the coming years. Valued at USD 84.18 billion in 2024, the market is expected to reach USD 109.92 billion by 2029, reflecting a robust compound annual growth rate (CAGR) of 5.48%.
Factors Driving Diagnostic Lab Industry Growth
Several factors propel the growth of the Diagnostic Labs Sector. Increasing disease prevalence, technological advancements, and heightened healthcare awareness among the populace are primary drivers. Additionally, the demand for clinical diagnostics remains buoyant, fueled by the emergence of mutant strains of the COVID-19 virus.
Major Players In The Diagnostic Labs Market
The Diagnostic Lab Market Major Players include industry stalwarts such as
Abbott Laboratories.
Bio-Rad Laboratories Inc.
Danaher Corporation
Becton, Dickinson
Qiagen
Roche Diagnostics.
These players wield significant influence, shaping the competitive landscape through innovation and market presence.
Trends Shaping the Diagnostic Labs Sector
One notable trend in the clinical diagnostics Sector is the increasing importance of lipid panel tests. These tests play a pivotal role in identifying individuals at risk of cardiovascular diseases, given the global prevalence of such conditions. The emphasis on early disease detection underscores the significance of lipid panel testing.
Regional Analysis of the Diagnostic Labs Market

North America emerges as a dominant force in the clinical diagnostics industry, driven by factors such as an aging population, heightened disease awareness, and a high prevalence of infectious and chronic diseases. The region's robust healthcare infrastructure and research funding further cement its market dominance.
Competitive Landscape of the Clinical Diagnostics Industry
The clinical diagnostics industry is fiercely competitive, with global and local players vying for market share. Companies like Abbott Laboratories and Roche Diagnostics lead the charge with innovative diagnostic solutions, driving industry advancements and addressing evolving healthcare needs.
Key Segments Covered
Revenue segmentation by government and private labs, pathology and radiology, pathology types, radiology types, and business models offers insights into the diverse facets of the diagnostic labs market, catering to varied healthcare requirements.
Key Target Audience
Government and private diagnostic labs
Research labs
Medical insurance companies
Medical device manufacturers
Industry associations
Government bodies
Investors/VCs constitute the key stakeholders shaping the diagnostic labs landscape.
Conclusion
In conclusion, the Diagnostic Labs market is experiencing robust growth, driven by factors such as increased disease prevalence, technological innovation, and rising healthcare awareness. With key players driving innovation and advancements, the future of clinical diagnostics looks promising, with a focus on improving disease detection and management.
FAQs
Q.1 What factors are driving the growth of the Diagnostic Labs Sector?
Ans. The growth is primarily driven by increasing disease prevalence, technological advancements, and rising healthcare awareness.
Q.2 Which regions are expected to dominate the clinical diagnostics market?
Ans. North America is anticipated to dominate the market, fueled by factors such as an aging population and a high prevalence of chronic diseases.
Q.3 What role do lipid panel tests play in clinical diagnostics?
Ans. Lipid panel tests are crucial for assessing cardiovascular risk and early disease detection, especially in the context of rising cardiovascular disease prevalence globally.
Q.4 Who are the major players in the Diagnostic Labs Industry?
Ans. Key players include Abbott Laboratories, Bio-Rad Laboratories Inc., Danaher Corporation, and Roche Diagnostics, among others.
Q.5 What is the future outlook for the clinical diagnostics market?
Ans. The market is poised for continued growth, driven by technological advancements, increasing disease burden, and healthcare infrastructure development.
#diagnosticlabmarket#diagnosticlabmarketsize#diagnosticlabmarkettrends#diagnosticlabmarketshare#diagnosticlabsector#diagnosticlabindustry#diagnosticlabmarketgrowthrate#diagnosticlabmarketrevenue#diagnosticlabmarketkeyplayers
0 notes
Text
We are the Trusted Choice in Medical Grade Cleaning Products
When it comes to healthcare, hygiene is not only preferred, but it is a must. The need for sanitary workplaces is critical, and using the best cleaning supplies is necessary to meet this requirement. Proudly made in the USA, Medical Grade Cleaning Products have become the first line of defence against pollutants and infections in hospitals and other healthcare settings.
The cornerstone of Medical Grade Cleaning Products lies in their unparalleled quality. Manufactured in state-of-the-art facilities across the United States, these products undergo rigorous testing and adhere to the strictest standards set forth by regulatory bodies. From Disinfecting wipes to Disinfecting sprays and Hand sanitizers, each formulation is meticulously crafted to deliver optimal results in healthcare settings.
Hospitals, where the stakes are highest, rely on the efficacy of these cleaning solutions to maintain a safe and sanitary environment for patients, staff, and visitors alike. With the constant influx of individuals carrying various pathogens, the need for robust cleaning protocols is non-negotiable. Medical Grade Cleaning Products like Sanitising wipes provide hospitals with the confidence they need to combat infectious agents effectively, mitigating the risk of healthcare-associated infections.
The efficacy of Medical Grade Cleaning Products extends beyond the confines of healthcare facilities. From schools and offices to residential homes, these products have garnered widespread acclaim for their ability to deliver consistent results. Families across the nation trust in the power of these cleaners to safeguard their loved ones against harmful germs and bacteria, providing peace of mind in an uncertain world.
In addition to their efficacy, Medical Grade Cleaning Products prioritize safety. Formulated with environmentally friendly ingredients, these cleaners offer a powerful yet gentle solution for disinfection. Unlike harsh chemicals that can pose risks to both users and the environment, these products strike the perfect balance between effectiveness and safety, ensuring that cleaning tasks can be performed without compromise.
The "Made in the USA" label carries with it a sense of pride and assurance. With Medical Grade Cleaning Products, this sentiment rings especially true. By choosing domestically produced cleaners, consumers not only support local economies but also invest in products that adhere to stringent quality control measures. The decision to manufacture these cleaning solutions within the USA underscores a commitment to excellence and accountability.
Beyond their primary function of disinfection, Medical Grade Cleaning Products embody a commitment to sustainability. By opting for eco-friendly formulations, these cleaners minimize their impact on the environment without sacrificing performance. This eco-conscious approach resonates with consumers who prioritize sustainability in their purchasing decisions, further solidifying the appeal of these products in the market.
The landscape of cleaning products is ever-evolving, driven by advancements in technology and science. Medical Grade Cleaning Products remain at the forefront of this innovation, constantly pushing the boundaries of what is possible in terms of efficacy and safety. Whether it's the development of new formulations or the integration of cutting-edge delivery systems, these products continue to raise the bar for cleanliness standards across industries.
The trust placed in Medical Grade Cleaning Products is not unfounded. Backed by scientific research and real-world testing, these cleaners have earned their reputation as reliable solutions for disinfection. Consumers can rest assured knowing that when they choose these products, they are choosing excellence – a sentiment echoed by hospitals, businesses, and households nationwide.
The widespread adoption of Medical Grade Cleaning Products serves as a testament to their success. From their humble beginnings to their current status as industry leaders, these cleaners have stood the test of time, proving their worth in the most demanding of environments. As the healthcare landscape continues to evolve, one thing remains constant: the indispensable role of Medical Grade Cleaning Products in safeguarding public health.
In an age where cleanliness is paramount, Medical Grade Cleaning Products stand as beacons of excellence. Trusted by hospitals and loved by everyone, these domestically produced cleaners embody a commitment to quality, safety, and innovation. Made in the USA, they serve as a testament to the nation's ability to lead the way in the fight against pathogens and contaminants. As we navigate the challenges of tomorrow, one thing is certain: Medical Grade Cleaning Products will continue to be indispensable allies in our quest for cleanliness and safety.
0 notes
Text
Middle East & Africa NGS Market - Opportunity Analysis And Industry Forecast (2024-2031)
Meticulous Research®—a leading global market research company, published a research report titled "Middle East & Africa NGS Market by Offering (Kits [Library Prep, QC, DNA Extraction], System), Type (Genome, Exome, Targeted), Application (Reproductive, Oncology, Infectious, Drug Discovery), Technology (SBS, Nanopore, Nanoball, SMRT Seq) – Forecast to 2031."
According to this latest publication from Meticulous Research®, the Middle East & Africa NGS market is expected to reach $356.5 million by 2031, at a CAGR of 11.1% from 2024 to 2031.The growth of the Middle East & Africa NGS market can be attributed to various factors, including the surge in genome sequencing programs, technological advancements in sequencing procedures, decreasing costs of genome sequencing, the rise in cancer prevalence, and the expanding applications of NGS in cancer treatment and research. However, the high costs of NGS systems and consumables and the availability of alternative technologies restrain the market's growth.
The rising adoption of bioinformatics and genomic data management solutions, as well as collaborations and partnerships to support next-generation sequencing, are expected to create market growth opportunities. However, the lack of skilled professionals and lack of standardization pose a significant challenge to the market's growth.
Key Players:
The key players profiled in the Middle East & Africa NGS market report are Thermo Fisher Scientific Inc. (U.S.), Illumina, Inc. (U.S.), Qiagen N.V. (Netherlands), F. Hoffmann-La Roche Ltd (Switzerland), PerkinElmer, Inc. (U.S.), Agilent Technologies, Inc. (U.S.), Danaher Corporation (U.S.), Bio-Rad Laboratories, Inc. (U.S.), Oxford Nanopore Technologies Plc. (U.K.), 10X Genomics, Inc. (U.S.), and Pacific Biosciences of California Inc. (U.S.).
Middle East & Africa NGS Market: Future Outlook
The Middle East & Africa NGS market is segmented into Offering (Consumables [Sample Preparation Consumables {DNA Extraction and Amplification, Library Preparation & Target Enrichment, Quality Control}, Other Consumables], NGS Platforms/Instruments, Software, Services), Sequencing Type (Targeted Genome Sequencing, Whole Genome Sequencing, Whole Exome Sequencing, Other Sequencing Types), Technology (Sequencing by Synthesis, Ion Semiconductor Sequencing, Single-molecule Real-time Sequencing (SMRT), Nanopore Sequencing, DNA Nanoball Sequencing), Applications (Clinical Applications [Reproductive Health, Oncology, Infectious Diseases, Other Clinical Applications], Research Applications [Drug Discovery, Agriculture & Animal Research, Other Research Applications]), End User (Hospitals and Diagnostic Laboratories, Pharmaceutical & Biotechnology Companies, Academic Institutes & Research Centers, Other End Users), and Geography.
Among the offerings, in 2024, the consumables segment is expected to account for the largest share of the Middle East & Africa NGS market. The large market share of this segment is attributed to the recurring use of consumables, the rising demand for NGS-based diagnostic tests, and increasing applications of NGS in oncology, reproductive health diagnosis, and drug discovery is increasing the demand for consumables
Among the sequencing types, in 2024, the targeted genome sequencing segment is expected to account for the largest share of the Middle East & Africa NGS market. The large market share of this segment is attributed to its low sequencing costs compared to other sequencing technologies, increased sensitivity in variant calling, and advancements in targeted genome sequencing technologies. Advances in targeted genome sequencing have enhanced the diagnosis of monogenic intestinal and pediatric disorders. Beyond disease diagnosis, this technology facilitates the exploration of gene-drug associations, enabling researchers to design gene-specific drugs with high specificity.
Among the technologies, in 2024, the sequencing by synthesis segment is expected to account for the largest share of the Middle East & Africa NGS market. The large market share of this segment is attributed to the advantages offered by sequencing by synthesis over other technologies. These advantages include the elimination of homopolymer errors, cost-effectiveness for various throughput or scale requirements, robust performance and data quality, unbiased coverage across the genome, and high-quality alignment achieved through paired-end sequencing.
Among the applications, in 2024, the research applications segment is expected to account for the larger share of the Middle East & Africa NGS market. The large market share of this segment is attributed to the increasing prevalence of genetic disorders, a rise in the adoption of sequencing-based tests in laboratories, the growing demand for gene-based medicines, increased investments in drug research and development activities, and increasing research programs for personalized medicine.
Among the end users, in 2024, the pharmaceutical & biotechnology companies’ segment is expected to account for the largest share of the Middle East & Africa NGS market. The large market share of this segment is attributed to the increasing adoption of advanced technologies for research purposes and increasing research for drug discovery with widening NGS applications, thereby accounting for the largest share of the market.
Geographic Review:
This research report provides a comprehensive analysis of the market in the United Arab Emirates, Qatar, Saudi Arabia, and the Rest of Middle East & Africa. In 2024, Saudi Arabia is expected to account for the largest share of the Middle East & Africa NGS market. Saudi Arabia’s significant market share can be attributed to the increasing adoption of advanced technologies for research purposes, the expanding applications of NGS in drug discovery, and growing research and development spending by pharmaceutical and biotechnology companies. These factors collectively drive the adoption of NGS technologies among pharmaceutical and biotechnology companies.
Download Sample Report Here @ https://www.meticulousresearch.com/download-sample-report/cp_id=5786
Key questions answered in the report:
Which are the high-growth market segments in terms of the offering, sequencing type, technology, application, end user, and country?
What was the historical market size for NGS in the Middle East & Africa?
What are the market forecasts and estimates for the period 2024–2031?
What are the major drivers, restraints, challenges, opportunities, and trends in the Middle East & Africa NGS market?
Who are the major players in the Middle East & Africa NGS market?
What is the competitive landscape like, and who are the market leaders in the Middle East & Africa NGS market?
What are the recent developments in the Middle East & Africa NGS market?
What are the different strategies adopted by the major players in the Middle East & Africa NGS market?
What are the geographical trends and high-growth countries?
Contact Us:
Meticulous Research®
Email- [email protected]
Contact Sales- +1-646-781-8004
Connect with us on LinkedIn- https://www.linkedin.com/company/meticulous-research
#MiddleEastndandAfricaNGSMarket#MassiveParallelSequencing#NextgenerationDNASequencing#TargetedSequencing#NGSSequencing#NGSTesting#NextGenerationSequencingMarket
0 notes
Text
DNA and RNA Sample Preparation Market Future Trends to Look Out | Bis Research
DNA and RNA sample preparation is a crucial step in molecular biology research, diagnostics, and various biotechnological applications. Proper sample preparation ensures the integrity, purity, and stability of nucleic acids, which are essential for downstream analyses such as PCR, sequencing, and gene expression studies.
The global nucleic acid sample preparation market is projected to reach $5,615.9 million by 2033 from $2,922.8 million in 2023, growing at a CAGR of 6.75% during the forecast period 2023-2033.
DNA and RNA Sample Preparation Overview
Understanding of the key principles and methods involved in preparing DNA and RNA samples for analysis Importance of Sample Preparation, Sample Collection, Extraction of nucleic acid, quality assessment, DNA preparation, RNA preparation, quality control storage prevention
Grab a look at the free sample page for more understanding click here !
Key Market Players
Agilent Technologies, Inc.
Autogen, Inc.
Bio-Rad Laboratories, Inc.
Roche AG
Merck KGaA
and many others
The Nucleic Acid Isolation and Purification Market has made an impact in the following ways:
Visit our Precision Medicine Vertical Page Click Here !
Market Segmentation
Product Type
End Users
Applications
Geography
Various different applications involved are as follows
Polymerase chain reaction
Sequencing
Gene Expression
Molecular Diagnostics
Epigenetics and Chromatin Analysis
Drug Discovery and Development
Market Drivers for DNA and RNA Sample Preparation Market
Advancements in Genomic Research
Rising Demand for Molecular Diagnostics
Increasing Applications in Drug Discovery and Development
Rising Incidence of Infectious Diseases and Genetic Disorders
Demand for Point-of-Care Testing (POCT) Solutions
Recent Developments in the Nucleic Acid Sample Preparation Market
Qiagen N.V. introduced two groundbreaking additions to its sample technologies portfolio, i.e., the TissueLyser III that facilitates high-throughput disruption of diverse biological samples and the RNeasy PowerMax Soil Pro Kit that isolates high-purity RNA from challenging soil samples using advanced Inhibitor Removal Technology.
Qiagen announced the launch of the QIAseq Normalizer Kits that give researchers a fast, convenient, and cost-effective method to pool different DNA libraries for best-quality results from next-generation sequencing (NGS) runs.
PerkinElmer introduced the CHEF Magnetic Bead Cleanup System, providing automated nucleic acid purification through advanced magnetic bead technology. This novel system would help automate the nucleic acid purification process efficiently.
PerkinElmer (Revvity, Inc.) rebranded its diagnostics and life sciences business as Revvity. This strategic move marked a new identity and focus for the company's ventures in these sectors.
Conclusion
As the demand for accurate, reliable, and efficient DNA and RNA sample preparation continues to rise, there is a growing emphasis on developing automated, integrated, and cost-effective solutions to meet the evolving needs of researchers, clinicians, and diagnostic laboratories. The convergence of cutting-edge technologies, strategic partnerships, and market expansion efforts is expected to drive the DNA and RNA sample preparation market forward, facilitating advancements in genomic medicine, precision diagnostics, and therapeutic development. Overall, the future of the DNA and RNA sample preparation market appears promising, with ample opportunities for innovation, growth, and impact across various sectors of the life sciences industry.
0 notes
Link
0 notes
Text
Transformative Technologies: In Vitro Diagnostics in Focus
IVD refer to medical devices and tests that are used to analyze samples taken from the human body, such as blood, urine, and tissue. These samples are collected from patients and tested outside of a living body in controlled laboratory conditions. IVD assists in disease screening, diagnosis of infections like HIV, monitoring disease progression or regression, and making decisions regarding drug treatments and medical interventions.
Growing Demand and Market Size
The global IVD market was valued at $70 billion in 2020 and is projected to reach $126 billion by 2028, expanding at a CAGR of 7.3% during the forecast period. The rising burden of chronic and infectious diseases, technological advancements in miniaturization and automation, point-of-care testing, and personalized medicine are some of the key factors driving the growth of the IVD industry. Precision medicine and companion diagnostics are also creating new opportunities for IVD manufacturers to cater to unmet medical needs.
Emerging Technologies
Some of the emerging technologies revolutionizing the In Vitro Diagnostics landscape include:
Next-Generation Sequencing (NGS)
NGS allows the sequencing of millions of DNA fragments simultaneously at high speed and low cost. It is being widely used for genetic disease screening, cancer diagnosis through tumor mutational burden testing, infectious disease detection, pharmacogenomics, and non-invasive prenatal testing. Continuous advancements in NGS workflow automation, data analysis, and interpretation are making it more accessible for clinical use.
Lab-on-a-Chip Technology
Also known as microfluidics, lab-on-a-chip miniaturizes traditional benchtop laboratory tests onto a silicon chip a few square centimeters in size. It allows automation and parallel processing of multiple diagnostic assays with minimal sample volume requirements. Applications include point-of-care testing for infectious diseases and glucose monitoring. Further advancement can make lab-on-chip diagnostics affordable for use in resource-limited settings.
Digital and Molecular Diagnostics
The digitization of diagnostic processes allows automation and streamlining of pre-analytical, analytical, and post-analytical stages. Digital PCR, isothermal amplification techniques, and microarray-based molecular diagnostics offer high sensitivity and specificity for infectious disease detection, genetic disorders screening, and cancer monitoring. Integration of AI and machine learning is augmenting data analysis capabilities.
Advancement in Biosensors
Continued research into nanotechnology, materials science, and sensor fabrication is revolutionizing the development of biosensors for IVD applications. Electrochemical, optical, and mass-sensitive biosensors enable rapid, multiplexed, affordable, and on-site testing with high precision. Applications include glucose monitoring, genetic disease screening, cardiac marker testing, infectious agent detection for epidemics and bioterrorism threats.
Challenges and Standardization Needs
While emerging technologies hold immense potential to transform diagnostics, their clinical validation and regulatory approval remain long drawn processes. Achieving standardization in pre-analytical variables, performance metrics, quality control protocols, and data interpretation across decentralized locations poses difficulties. High initial investment and operational costs can delay the real-world adoption of advanced IVD technologies, especially in low to middle-income countries. Lack of skilled labor and infrastructure in resource-limited regions further hampers access to quality diagnostic services. Overcoming these challenges through partnerships, standardized guidelines, innovative business models, and human capital investments would be crucial to realize the full benefits of emerging IVD technologies.
Regulatory Changes and Global Harmonization
In vitro diagnostic regulators worldwide are aligning processes and requirements to facilitate the global development and distribution of new IVD technologies. The U.S. FDA is shifting from a risk-based to a total-product lifecycle approach through the implementation of the Verification and Validation framework. The European IVD Regulation establishes a single regulatory structure across EU markets. Global harmonization initiatives led by bodies like the World Health Organization aim to establish consistent standards and mutual recognition of approvals. Such regulatory changes intend to expedite patients' access to advanced diagnostics while maintaining pre-market evaluation of safety, efficacy, and performance.
Future Trends and Conclusion
The future of IVD looks promising with advancements spanning multiple omics technologies, digital platforms, lab miniaturization, and big data analytics. Integration of diagnostics into therapeutic strategies will become more prevalent. Radical new technologies like mobile health diagnostics, wearable biosensors, and molecular pathology could transform healthcare delivery models. Nonetheless, building robust research infrastructure, streamlining regulatory pathways, ensuring affordability, and addressing ethical issues would be pre-requisites to realize the full potential. IVD's crucial role in public health interventions and precision medicine will continue propelling innovations aimed at making diagnostics more accessible, non-invasive, rapid, accurate, and cost-effective.
0 notes