#Hepatocellular Carcinoma Drug Market
Text
Yangzheng Xiaoji Capsules: Adjuvant Therapy for Tumors
Yiling Pharmaceutical is a traditional Chinese medicine enterprise whose main business is the R & D, production and sales of patented innovative traditional Chinese medicine, and at the same time actively distributes the chemical and health industries, forming a medical and health industry pattern of coordinated development and mutual promotion of patented traditional Chinese medicine, chemical medicine and health industry.
In 1992, the predecessor of Ling Pharmaceutical Industry, the Medical and Pharmaceutical Research Institute of Shijiazhuang Development Zone and Huangdi Pharmaceutical Factory were established. In 2001, after the joint-stock reform, Yiling Pharmaceutical was officially established. The company has always adhered to the collateral disease theory as the basis, always adhere to the research and development and innovation of patented new drugs, successfully developed and listed Tongxinluo capsule, Yangzheng Xiaoji capsule, Lianhua Qingwen capsule and other important products.
Yiling Pharmaceutical Co., Ltd. focuses on patented new drugs and gradually forms a rich product echelon. Under the guidance of innovative collateral disease theory, the company has successfully issued a series of patented traditional Chinese medicine with independent intellectual property rights around six major disease areas with high incidence and large market consumption, such as cardio-cerebrovascular disease, diabetes, cold breathing, tumor, nerve, urinary and so on.
Yangzheng Xiaoji capsule is used in the adjuvant treatment of malignant tumor and is included in the guidelines for cancer diagnosis and treatment. Yangzheng Xiaoji capsule put forward for the first time the drug principle of "Sanjie Tongluo" in the treatment of tumor, which has the functions of inhibiting tumor angiogenesis and tumor cell metastasis. Combined with radiotherapy and chemotherapy can reduce toxicity and increase efficiency, regulate immunity and improve the quality of life of tumor patients. It has become an important drug for rehabilitation and palliative treatment of tumor patients. Yangzheng Xiaoji buy online is also very convenient.
According to the company's annual report, a randomized, double-blind, multi-center clinical study led by Guang'anmen Hospital of China Academy of Traditional Chinese Medicine and Beijing Cancer Hospital has confirmed that Yangzheng Xiaoji Capsules can improve the efficacy of chemotherapy in treating the solid tumors of primary hepatocellular carcinoma, reduce the inhibition of chemotherapy on hemoglobin, leukocytes, and platelets, and elevate the NK cells; alleviate the damage of liver function, and improve the fatigue and anorexia associated with cancer to improve the quality of life of the patients. Quality of Survival. Research conducted by Cardiff University and Beijing Cancer Hospital confirmed that Yangzheng Xiaoji capsule reduces the resistance of molecularly targeted drugs to EGFR therapy, which means that the efficacy of molecularly targeted drugs is expected to be further improved by combining Yangzheng Xiaoji capsule with other drugs. Since 2020, the Nourishing Zheng elimination capsule has been successively incorporated into the "China Cancer-related Fatigue Clinical Practice Guidelines (2020)", "China Tumor Psychology Clinical Practice Guidelines (2020)" and "Breast Cancer Combined Western and Traditional Chinese Medicine Specialist Diagnostic and Treatment Consensus (2020)", and it is expected to enter the period of rapid volume release.

0 notes
Text
Hepatitis Drugs Market Expansion: A Hopeful Horizon for Liver Health
Introduction
The global hepatitis drugs market has witnessed significant advancements and opportunities in recent years. Hepatitis, a viral infection that affects the liver, has been a major public health concern worldwide. With the advent of innovative pharmaceuticals and a deeper understanding of the hepatitis virus, the market for hepatitis drugs has expanded, offering new hope to millions of individuals affected by this disease. This article delves into the current state of the hepatitis drugs market, the key players, and the promising developments that are shaping the industry.
Hepatitis Overview
Hepatitis is a group of infectious diseases caused by hepatitis viruses, including A, B, C, D, and E. Among these, hepatitis B and C are the most common and serious, often leading to chronic liver disease, cirrhosis, and hepatocellular carcinoma. Over the years, extensive research and development have led to the discovery of various antiviral medications that target these infections.
Market Size and Growth
The hepatitis drugs market has experienced robust growth in recent years. The rising prevalence of hepatitis infections, increased awareness about the disease, and the availability of improved diagnostic techniques have all contributed to the expansion of this market. As of my last knowledge update in early 2022, the global hepatitis drugs market was estimated to be worth billions of dollars, and it is expected to continue growing in the coming years.
Key Players
Several pharmaceutical companies are actively involved in the development and commercialization of hepatitis drugs. Gilead Sciences, AbbVie, and GlaxoSmithKline are among the major players in this market. Gilead Sciences, in particular, has made a significant impact with its hepatitis C drugs, including sofosbuvir and ledipasvir, which have revolutionized the treatment of this infection.
Advancements in Hepatitis Drugs
Recent advancements in hepatitis drugs have brought about a shift in the treatment landscape. The introduction of direct-acting antivirals (DAAs) for hepatitis C has significantly improved cure rates and reduced treatment duration. These medications have fewer side effects compared to older therapies, making them more accessible to a broader patient population. Furthermore, the development of combination therapies has increased the likelihood of a sustained virologic response, meaning patients can achieve a cure from hepatitis C.
Hepatitis B treatment has also seen noteworthy progress. Nucleoside/nucleotide analogs (NAs) such as tenofovir and entecavir are now commonly used to suppress the replication of the hepatitis B virus. These drugs help manage the disease and prevent liver damage, although they may not provide a complete cure.
Challenges and Opportunities
Despite the positive developments in hepatitis drugs, challenges remain. Access to these medications can be a hurdle, particularly in low- and middle-income countries. The high cost of newer hepatitis C drugs, in particular, has been a point of concern. Ensuring equitable access to these life-saving treatments is a priority.
Opportunities in the hepatitis drugs market are driven by ongoing research and development efforts. Scientists are continually exploring new therapeutic targets and potential vaccines for hepatitis B. Improved diagnostic methods and increased vaccination efforts for hepatitis A and B are also essential components of reducing the disease burden.
Conclusion
The hepatitis drugs market has come a long way in the treatment of hepatitis B and C infections. With advancements in drug development, there is renewed hope for individuals living with these conditions. While challenges related to access and affordability persist, the ongoing research and development in this field offer a promising outlook for the future. As healthcare systems and governments worldwide focus on eradicating hepatitis, the market for hepatitis drugs will continue to play a crucial role in achieving this goal.
Few Other Promising Reports in Pharmaceutical Industry
Global Major Depressive Disorder (MDD) Treatment Market
Neuromodulation Devices Market
Mental Disorder Treatment Market
Serotonin Norepinephrine Inhibitor Market
0 notes
Text
Current Advancements in HCC Diagnosis & Treatment in South-East Asian Countries
Hepatocellular carcinoma, or HCC, the most common liver cancer type, is a significant public health concern in South-East Asian countries. Over the past few years, remarkable advancements have been made in HCC diagnosis and treatment, offering new hope to patients and healthcare providers. This blog discusses the current state of HCC diagnosis and treatment in South-East Asian countries, highlighting the latest technologies and approaches that have improved patient outcomes.
Write to us at [email protected] Learn how GRG Health is helping clients gather more in-depth market-level information on such topics
Advanced Diagnostic Techniques:
Liquid Biopsy:
Liquid biopsy has gained popularity as a promising non-invasive diagnostic tool for HCC detection in South-East Asia. By analyzing circulating tumor DNA and other biomarkers in blood samples, liquid biopsy enables early detection, monitoring of treatment response, and identifying potential relapse in HCC patients. Its convenience and accuracy make it valuable to traditional imaging and biopsy methods.
Imaging Advancements:
South-East Asian countries have embraced cutting-edge imaging technologies, such as contrast-enhanced ultrasound (CEUS) and dynamic contrast-enhanced magnetic resonance imaging (MRI). These techniques offer higher sensitivity and specificity in detecting small liver lesions, aiding in early-stage HCC diagnosis and precise treatment planning.
Personalized Treatment Approaches:
Targeted Therapies:
Advancements in targeted therapies have revolutionized HCC treatment in South-East Asia. Sorafenib and lenvatinib are among the FDA-approved targeted drugs that inhibit specific molecular pathways responsible for tumor growth. Their introduction has improved survival rates and provided viable treatment options for patients with advanced-stage HCC.
Immunotherapy:
Immunotherapy, specifically immune checkpoint inhibitors, such as nivolumab and pembrolizumab, has shown promising results in HCC patients. These drugs stimulate the immune system to recognize and attack cancer cells, offering new possibilities for patients who previously had limited treatment options.
Minimally Invasive Interventions:
Radiofrequency Ablation (RFA):
RFA has become a widely adopted treatment option in South-East Asian countries, particularly for early-stage HCC patients. Using thermal energy to destroy cancer cells, RFA is a minimally invasive procedure that preserves healthy liver tissue and results in faster patient recovery times.
Transarterial Chemoembolization (TACE):
TACE is an interventional radiology technique used to deliver chemotherapy directly to the tumor site, cutting off its blood supply. This procedure effectively manages intermediate-stage HCC, slows tumor progression, and improves overall survival rates.
Multi-disciplinary Care:
South-East Asian countries have recognized the importance of a multi-disciplinary approach in managing HCC. Comprehensive cancer centers bring together oncologists, hepatologists, radiologists, surgeons, and other specialists to develop personalized treatment plans for each patient. This collaboration ensures the best possible outcome and a holistic approach to HCC care.
Conclusion:
HCC diagnosis and treatment in South-East Asia have improved with liquid biopsy and advanced imaging techniques, leading to better patient outcomes. Targeted therapies, immunotherapies, and minimally invasive interventions have extended survival rates and reduced patient burden. Multi-disciplinary care ensures comprehensive treatment and ongoing research and technology advancements offer hope for even more effective approaches to combat HCC. South-East Asian countries are making significant strides toward improving patient outcomes and enhancing the quality of life.
0 notes
Text
0 notes
Text
Hepatocellular Carcinoma Market demand will reach a value of US$ 41.57 Billion by the year 2030 at a CAGR of 11.5% | Growth Plus Reports
Newark, New Castle, USA – Growth Plus Reports’ most recent study examines the Global Hepatocellular Carcinoma Market’s production, prospective uses, demand, key companies, and SWOT analysis.

You may get insights into the TOC, and Statistics for essential facts, information, trends, and competitive landscape information.
Download Free Sample Report Now @ https://www.growthplusreports.com/inquiry/request-sample/hepatocellular-carcinoma-market/8027
The following are the leading companies in the Global Hepatocellular Carcinoma market:
Bayer AG
Bristol-Myers Squibb
Genentech Inc.
Eisai Inc.
Merch & Co. Inc.
Eli Lilly & Company
Dainippon Sumitomo Pharma
Growth Plus Reports studies the key trends in each category and sub-segment of the Hepatocellular Carcinoma market, along with Global and regional projections from 2023 to 2031. Our research splits the market into product type and application segments.
SEGMENTATION
GLOBAL HEPATOCELLULAR CARCINOMA MARKET – ANALYSIS & FORECAST, BY MARKETED DRUG
Sorafenib (Nexavar)
Nivolumab (Opdivo)
Bevacizumab (Avastin)
Regorafenib (Stivarga)
Lenvatinib Mesylate (Lenvima)
Pembrolizumab (Keytruda)
Atezolizumab (Tecentriq)
Cabozantinib (Cabometyx)
Ramucirumab (Cyramza)
Miriplatin (Miripla)
For More Information or Query or Customization visit: https://www.growthplusreports.com/inquiry/customization/hepatocellular-carcinoma-market/8027
Companies may utilize Hepatocellular Carcinoma market report to get insights on market variables and any restraints that may affect the manufacturing of their product. Companies that are expanding abroad require thorough Global market research that includes real market data to assist with their marketing strategy. This Global market Hepatocellular Carcinoma industry study analyzes important market dynamics and provides in-depth information and statistics to help companies flourish. This research report on the Hepatocellular Carcinoma market takes advantage of advanced and professional approaches such as SWOT analysis and GRG Health’s unique GrowthMIX strategy.
This report is useful in addressing various essential issues for market participants, while also supporting them in making investments and leveraging the market opportunities.
Hepatocellular Carcinoma Market TOC: https://www.growthplusreports.com/report/toc/hepatocellular-carcinoma-market/8027
Market segment by Region/Country including: –
-North America (United States, Canada and Mexico)
-Europe (Germany, UK, France, Italy, Russia and Spain etc.)
-Asia-Pacific (China, Japan, Korea, India, Australia and Southeast Asia etc.)
-South America (Brazil, Argentina and Colombia etc.)
-Middle East and Africa (South Africa, UAE and Saudi Arabia etc.)
QUICK BUY: https://www.growthplusreports.com/checkout-8027
Browse Latest Healthcare Reports:
MOG Antibody Associated Disorders Treatment Market
Aortic Valve Replacement Devices Market
Alveolar Soft Part Sarcoma Market
Magnetic Resonance Imaging Equipment Market
Facet Joint Injections Market
Health Telemetry Market
Biotechnology Market
Hernia Repair Market
Endotoxin Testing Market
Transcriptomics Market
0 notes
Text
0 notes
Text
Non-Alcoholic Steatohepatitis Clinical Trials Market: Global Industry Analysis, Size, Share, Growth, Trends And Forecast 2022 to 2032
According to Future Market Insights’ Non-Alcoholic Steatohepatitis Clinical Trials Market Study research, global sales were stable at US$ 2.5 billion in 2021. The anticipated market growth from 2022 to 2032 is anticipated to be 7%, which is much greater than the increase in the past. With a CAGR of roughly 11.2% from 2022 to 2032, Phase 3 is anticipated to generate the largest revenue for the Non-Alcoholic Steatohepatitis Clinical Trials Market.
Market for Non-Alcoholic Steatohepatitis Clinical Trials from 2017 to 2021 in terms of revenue In contrast to the Demand Forecast for 2022 to 2032
According to a study on the Non-Alcoholic Steatohepatitis Clinical Trials Market conducted by Future Market Insights, a provider of market research and competitive intelligence, from 2017 to 2021, the market value of this disease increased at a compound annual growth rate (CAGR) of about 6.2%, with the USA, the UK, China, and Japan accounting for a sizable portion of the global market.
Request Sample @
https://www.futuremarketinsights.com/reports/sample/rep-gb-15802
Moreover, advancing technology, an ageing population, an increase in the prevalence of chronic diseases, and an increase in surgical operations are the main drivers of the expansion. Because of this, the market for non-alcoholic steatohepatitis clinical trials is anticipated to expand at a CAGR.
How did the market for non-alcohol steatohepatitis clinical trials perform during the pandemic?
Trials were put on hold as a result of the pandemic because of decreased patient participation in clinical research and supply chain disruptions. Virtual participants and COVID-compliant screening, however, allowed some organisations to complete the trials. As an illustration, Novartis conducted a phase two trial of a particular medicine for non-alcoholic steatohepatitis after the drug was recognised as a ground-breaking treatment in the United States. In phase 3a, semaglutide will be started in patients with non-alcoholic steatohepatitis (NASH) in 2021. The outcomes of a phase two proof-of-concept experiment in NASH were given by Novo Nordisk and Gilead Sciences. In the US, NASH is the second most common reason for liver transplants. Hepatocellular carcinoma is also connected to NASH.
The results of a phase two proof-of-concept study in NASH were revealed by Nordisk and Gilead Sciences. The second most common reason for liver transplants in the US is NASH. Several studies have found a connection between NASH and hepatocellular cancer growth.
Ask An Analyst @
https://www.futuremarketinsights.com/ask-question/rep-gb-15802
Analysis by country
The USA Clinical Trials for Non-Alcoholic Steatohepatitis: Market Research
One of the most prevalent chronic liver diseases in the USA is NAFLD. In the USA, roughly 20% of adults are thought to have NASH and about 25% are thought to have NAFLD. Given these worrying figures, the Non-Alcoholic Steatohepatitis Clinical Trials market in the USA is predicted to grow at a CAGR of 7.5% from 2022 to 2032, reaching a valuation of US$ 1.9 billion.
It can be challenging to separate patients into distinct fibrosis phases in NASH trials because to pathologist bias. In order to achieve more accurate and consistent biopsy analysis, Sagimet Biosciences is adopting digital histopathology in its Stage IIb non-alcoholic steatohepatitis (NASH) investigation. Digital pathology for NASH involves histological imaging of biopsy samples, which are subsequently assessed using artificial intelligence (AI) to find fibrosis alterations. Sagimet is collaborating with HistoIndex, a Singapore-based diagnostic company, in the Phase IIb FASCINATE-2 trial (NCT04906421) to research TVB-2640.
Market Segments Covered in Non-Alcoholic Steatohepatitis Clinical Trials Market Analysis
By Study Design:
Interventional
Observational
Expanded Access
By Phase:
Phase 1
Phase 2
Phase 3
Phase 4
Request Customization @
https://www.futuremarketinsights.com/customization-available/rep-gb-15802
By Region:
North America
Latin America
Asia-Pacific (APAC)
Middle East and Africa (MEA)
Europe
0 notes
Text
Increasing prevalence of cancer is supporting the growth of the global recombinant human endostatin market

North America is expected to lead the global recombinant human endostatin market and this is attributed to the high prevalence of cancer in the region which is creating demand for such therapy. According to a report by the Centers for Disease Control and Prevention (CDC), around 1,633,390 new cancer cases were reported in the U.S. in 2015 and the number is expected to reach 1,735,350 in 2018. Also, an increasing number of clinical trials is again expected to foster the growth of the market.
Key players operating in the global recombinant human endostatin market include Pfizer Inc., Novus Biologicals, Biocon, Thermo Fisher Scientific, Yantai Medgenn Ltd., FUJIFILM KYOWA KIRIN BIOLOGICS Co., Ltd., PeproTech, Inc., Onyx Pharmaceuticals, Inc., Genexine, Inc., Hetero., Intas Pharmaceuticals Ltd., and Novartis AG.
A growing number of research & development activities to recognize potential applications of recombinant human endostatin is expected to foster the growth of the recombinant human endostatin market. Increasing demand for cancer treatment and ongoing studies to identify potential collagen are again fostering the growth of the market. According to the World Health Organization (WHO), around 18.1 million new cases of cancer were recorded in 2018, with 9.6 million deaths due to the disease. Also, the rise in the geriatric population around the globe coupled with the expanding pharmaceutical & biotechnology companies using recombinant human endostatin is further projected to foster the growth of the market.
Moreover, increasing investment in the research &development activities based on the use of recombinant technology is further anticipated to augment the growth of the market.
In January 2017, the PLA General Hospital in China has undertaken a clinical phase II study for recombinant human endostatin combination with radiotherapy for the treatment of Hepatocellular Carcinoma (HCC). The study was estimated to complete in December 2019.
A clinical trial is a rigorous medical procedure intended to test an important new medical product or procedure. The goal of such a trial is to provide evidence for or against the effectiveness, safety, and effectiveness of medical treatment. Clinical trials are conducted by healthcare companies to test drugs, medical devices, and other health products. Sometimes, healthcare companies conduct both clinical trials and consumer-based tests to determine whether a new product's benefits are worth the cost of introducing it into the marketplace. For Recombinant Human Endostatin, a biotechnology company called Generex has conducted an important clinical trial.
0 notes
Text
PERFORMANCE ASSESSMENT OF BLOOD SCREENING ASSAYS: EARLY DYNAMICS OF HEPATITIS B VIRUS (HBV)-DNA AND SURFACE ANTIGEN (HBSAG) IN RAMP-UP PHASE OF VIREMIA
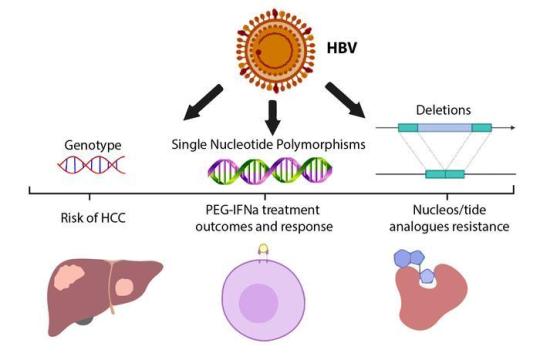
In a previous article, we discussed Hepatitis C, a serious liver disease caused by the hepatitis C virus (HCV), and the dangers of HCV and HIV co-infection. Today's topic is Hepatitis B, which is a liver infection caused by the hepatitis B virus (HBV). Hepatitis B is a viral infection that affects the liver and can cause both acute and chronic disease. Acute Hepatitis B can last for as little as six months. In its chronic form, the virus remains in the person's body for a longer period of time and goes untreated; it can endanger people's lives by causing cirrhosis, fibrosis, hepatocellular carcinoma, and end-stage liver disease.
Hepatitis B has no known cure. In some cases, the infection will resolve on its own (in 4 to 8 weeks for more than 9 out of 10 adults). Some adults who contract this virus as adults become "carriers," (a chronic condition) and are likely to infect others for the rest of their lives unknowingly. CHB patients must be treated for the rest of their lives. Early HBV exposure frequently results in chronic HBV and the virus's persistence due to the lack of cellular immune responses or strong antibodies.
HBV carriers may benefit from treatment that inhibits viral replication, boosts immune defences against the virus, or a combination of the two. The effectiveness of the treatments is entirely dependent on regular testing and monitoring of the various stages of the disease. One of the blood tests used to determine viral activity in an infected patient's body is the HBV DNA (viral load test).
The natural progression of chronic HBV infection can be divided into five stages, the last of which is seroconversion of Hepatitis B Surface Antigen. Hepatitis B is not detected in your blood at this stage, or hepatitis B surface antigen (HBsAg) is negative or not detected in the blood.
Helvetica Health Care is a market leader in the provision of seroconversion panels and blood screening assays, which are critical in the monitoring and treatment of life-threatening diseases such as Hepatitis B, Hepatitis C, and HIV. We hope that the information provided below will help you learn more about HBV and the dynamics of the Hepatitis B Virus (HBV)-DNA viral load test and Surface Antigen (HBsAg) in the Ramp-Up Phase of Viremia (the presence of virus in the blood).
WHAT IS THE HBV-DNA VIRAL LOAD TEST?
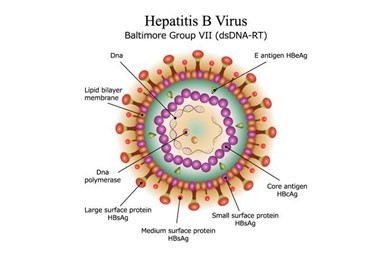
The viral load test, also known as hepatitis B virus DNA quantification, is a blood test that determines the amount of hepatitis B virus DNA in a chronically infected patient's blood. Viremia is typically measured in "international units per millilitre" (IU/mL); previously, it was measured in "copies per millilitre" (cp/ml). The Polymerase Chain Reaction (PCR) technique is used for the test.
Other factors, such as Hepatitis B e-antigen (HBeAg) status, liver enzymes or serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT) tests, and inflammation levels, must support the information gathered through this test. It is critical to monitor your plasma viral load on a regular basis in order to determine the stage of your infection. Several lab tests, including viral load, are used to determine the stage of the infection.
In chronic HBV infection, quantitative understanding of HBV dynamics influences drug treatment and immunotherapy timing. It can aid in the development of the best treatment plans for individual patients.
The five stages of Hepatitis B infection are
Immune tolerance,
Immune active,
Inactive HBV carrier state,
HBeAg-negative chronic hepatitis B, and
Hepatitis B surface antigen (HBsAg)-negative
Chronic hepatitis B (CHB) is when hepatitis B surface antigen (HBsAg) persists for six months or more. Most infected persons are unaware of their HBV infection and present advanced disease.
Annually, between 0·5% and 3% of inactive HBV carriers lose HBsAg. Spontaneous HBsAg clearance usually confers a good prognosis if there is no pre-existing hepatocellular carcinoma or cirrhosis at the time of HBsAg seroclearance (the clearance or removal of an antigen from the blood.) Following the loss of HBsAg, seroconversion to antibody against hepatitis B surface antigen (anti-HBs) is more likely to stop the development of cirrhosis and hepatocellular cancer, and it shows immunity to HBV and may suggest a better prognosis.
Throughout treatment, it is critical to monitor HBV DNA levels. Doctors detect HBV DNA when administering daily antiviral medications to see if the drug is lowering your viral load and to ensure that the antiviral is effective.
Testing of the International Standard and 10-30 seroconversion panels is required to demonstrate "state of the art" assay performance for detecting bloodborne viruses such as HBV. The evaluation of HBV-DNA and HBsAg assay performance is critical for understanding the dynamics of Hepatitis B Virus (HBV)-DNA and Surface Antigen (HBsAg) in the Ramp-Up Phase of Viremia. According to research, the viral doubling duration in the ramp-up phase (period of exponential growth in viral load) is the same above and below the quantification limit of the viral load assay.
It is critical to avoid hepatitis B infection in order to reduce the risk of developing chronic disease or liver cancer. Regular testing that yields high-quality and accurate results is the only way to prevent disease. Ensuring high-quality test results is not only good for public health but also good for your lab's reputation.
HHC's QUALITY CONTROL PANELS can be used to test the sensitivity, specificity, and working range of your assays, as well as for diagnostic development and batch release in manufacturing. Panels include representative data from current market assays. Depending on the intended use of the panel, our VERIFICATION / VALIDATION Panels are designed to be used with assays to determine the presence of antigen, antibody, RNA, or DNA.
In addition, we have an extensive range of SEROCONVERSION PANELS for detecting asymptomatic donors infected with HIV, HCV, HBV and EBV, and SURVEILLANCE PANELS and LONGITUDINAL PANELS. Our panels are run on as many different diagnostic kits as possible to measure relevant markers of seroconversion. All testing is performed by Certified Reference Laboratories and Domestic and International Regulatory Bodies.For more information, contact us now!
0 notes
Text
Fourth-generation chimeric antigen receptor T Cells for hepatocellular carcinoma
Results for multiple hepatocellular carcinoma (HCC) clinical trials were presented at the 2021 American Society of Clinical Oncology (ASCO) virtual yearly meeting held June 4-8 June. CARsgen Therapeutics presented data from a Stage I trial (NCT03980288) of its fourth-generation fanciful antigen receptor T (Vehicle T) cells targeting glypican-3 (GPC3). Fourth-generation Vehicle T cells are additionally designed to secrete a transgenic cytokine upon Vehicle motioning in the targeted tumor tissue. As a result, in addition to the direct antitumor attack, they can trigger T cells to eliminate antigen-negative malignant growth cells at the target site. GPC3 is a cell-Cells Expressing T Cell Surface Glycoprotein CD5 Drugs Development Market that is profoundly communicated in HCC and is thus being evaluated as a target for HCC immunotherapy. CARsgen's original pipeline therapy for cutting edge HCC stands out for being a first-in-class approach with an extraordinary system of action that targets vigorously pretreated progressed HCC patients.
In this study, CARSgen's fourth-generation Vehicle GPC3 T cells in combination with the tyrosine kinase inhibitors (TKIs) Nexavar (sorafenib) or Stivarga (regorafenib) demonstrated sensible safety and potential antitumor activity for hepatitis B infection (HBV)- related metastatic HCC patients who have been recently treated with systemic therapy. Safety results showed a shortfall of portion limiting toxicity, treatment-related serious unfavorable events (AEs), AE-related death or study withdrawal, and neurotoxicity. Three out of the six patients in the study experienced grade 3 cytokine discharge condition (CRS), a typical AE found because of Vehicle T cell therapy. Fundamental viability results uncovered the objective reaction rate and infectious prevention rate were 16.7% and half, respectively. The middle movement free endurance was 4.2 months. The middle pinnacle Vehicle GPC3 duplicate number in patients' fringe blood on day 7 after treatment was 5,067 duplicates for each μg genomic DNA and Vehicle GPC3 duplicates were still detectable on day 28 at levels running between 113 and 2,071 duplicates for every μg genomic DNA. Prior Stage I trials for the second-generation variant of this therapeutic agent (NCT02395250 and NCT03146234) likewise reported clinical benefit and tolerability.
KOLs interviewed by GlobalData noted that Vehicle T cells or adoptive cellular therapy is an arising region that might change HCC treatment practice from here on out. The majority of late-stage pipeline drugs for cutting edge HCC are for use in the first-line setting, which has the highest number of treated cases. Therefore, subsequent lines of therapy are being neglected by drug designers, despite high unmet need. Notwithstanding, CARsgen Therapeutics conducted the study of their Vehicle T cell therapy on patients who advanced on at least two lines of systemic therapy, having tried at least one TKI joined with anti-modified death-1 (PD-1)/customized death ligand 1 (PD-L1) immunotherapy or FOLFOX4 (5-fluorouracil, leucovorin, and oxaliplatin) chemotherapy. A comparative strategy of zeroing in on subsequent lines of therapy was utilized by other organizations studying adoptive cellular therapy for HCC in the beginning phase development including Immunotech Biopharm, Immunicum Stomach muscle, Aha Therapeutics, Adaptimmune Therapeutics, Fate Therapeutics, and Origincell Therapeutics. Therefore, investigation of vigorously pretreated progressed HCC patients is by all accounts a general trend with Vehicle T cell designers in HCC.
The HCC clinical pipeline is packed with me-too drugs targeting patients in the first-line setting. CARsgen's Vehicle GPC3 T cell therapy is a first-in-class targeted drug tending to cutting edge HCC patients who have advanced on or created resistance to past therapy — a patient population with little to no treatment options. GlobalData expects that assuming effective in later stage trials, CARsgen's therapy will be advantageous to underserved patients in late-stage progressed HCC.
Cell and Quality Therapy Inclusion on Clinical Trials Field supported by Cytiva.
Editorial content is independently created and keeps the highest guidelines of journalistic integrity. Topic supports are not associated with the creation of editorial content.
0 notes
Text
Research Nester published a report titled “Liver Cancer Therapeutics Market: Global Demand Analysis & Opportunity Outlook 2031” which delivers detailed overview of liver cancer therapeutics in terms of market segmentation by type, therapy, and by region.
Further, for the in-depth analysis, the report encompasses the industry growth indicators, restraints, supply and demand risk, along with detailed discussion on current and future market trends that are associated with the growth of the market.
The global of liver cancer therapeutics market is estimated to garner significant revenue by growing at a CAGR of 20.2% over the forecast period, i.e., 2022 – 2031. The growth of the market can be attributed to growing prevalence of liver cancer, and rise in incidence of hepatitis B infection in developing countries. According to the World Health Organisation, liver cancer was ranked third in the most common causes of cancer death in the world in 2020, causing nearly 830,000 deaths, while in 2019, hepatitis B resulted in an estimated 820,000 deaths globally. The market is segmented by type into hepatocellular carcinoma, cholangiocarcinoma, hepatoblastoma, and others, out of which, the hepatocellular carcinoma segment is anticipated to hold the largest share in the global liver cancer therapeutics market over the forecast period. In 2018, there were an estimated number of 661,000 cases of hepatocellular carcinoma recorded worldwide, that contributed 80% of the world total liver cancer burden.
Geographically, the global liver cancer therapeutics market is segmented into five major regions including North America, Europe, Asia Pacific, Latin America and Middle East & Africa region, out of which, North America is anticipated to hold the largest market share owing to high awareness about liver cancer, presence of well-established healthcare infrastructure, and easy drug availability. Additionally, Asia-Pacific region is estimated to witness growth at the highest rate during the forecast period.
In Q4 2021, USA current-account deficit widened stood at $224.8 billion. However, in Q1 22, CAD rose by 29.6%, reached to $291.4 billion, adding $66.6 billion to the gap. Export of good and services increased by $25.7 billion to reach $1.03 trillion in the first quarter of 2022. However, goods and services deficit was $79.6 billion in June, down $5.3 billion from $84.9 billion in May, revised- reflecting some sight of relief. On the other hand, annual inflation rate in the country hit 8.5%. Energy CPI surged by 32.9% in July 2022, inflating the cost of logistic and some signs to disrupt supply chain whilst electricity cost upsurged by 15.2%, highest since Feb 2006. Apart from that, In July 2022, existing US home sales declined 5.9% to 4.81 million (seasonally adjusted annual rate), the lowest since May of 2020 and below market expectations of 4.89 million. As mortgage rate touches to peak 6%, sales for houses declined for a sixth consecutive month. Global energy crises to remain at focal point, pushing consumers to spend less on the products and services and save more.
On the other hand, the worst is expected to be seen in the European countries especially during 2022 winters. The energy and gas crises has already started grappling the region where in many Western European countries including Germany is looking for coal fired solutions to tackle the gas supply shortage, created by Russian-Ukraine conflict.
Amidst global concerns, market players have started looking for safe investments by holding on to the new technology and product launches. Factors like currency translation, disruption in global supply chain, Anti-China sentiments brewing across the globe, slowdown in Chinese economy, inflated products prices, USD getting stronger every week, decreasing purchasing power and strict measures taken by central banks/institutions across the world to ensure less spending and more saving, could hit the demand for the product and service badly in near future.
Healthcare Companies and Private Service Providers to have a minimal damage Caused by Inflation:
As US govt. remains committed to quality by spending more in the Medicaid and Medicare programs, incentives by govt. to medical devices, pharma companies and biotech to benefit the market players in short and long term goals. In 2021, U.S. spent $12,318/person on healthcare- highest amongst all OECD countries followed by Germany at $7,383. The federal government commitment towards healthcare systems to enable market players expanding their revenues and mitigating the risk posed by the inflation.
Download Sample of This Strategic Report @ https://www.researchnester.com/sample-request-4114
The research is global in nature and covers detailed analysis on the market in North America (U.S., Canada), Europe (U.K., Germany, France, Italy, Spain, Hungary, Belgium, Netherlands & Luxembourg, NORDIC [Finland, Sweden, Norway, Denmark], Poland, Turkey, Russia, Rest of Europe), Latin America (Brazil, Mexico, Argentina, Rest of Latin America), Asia-Pacific (China, India, Japan, South Korea, Indonesia, Singapore, Malaysia, Australia, New Zealand, Rest of Asia-Pacific), Middle East and Africa (Israel, GCC [Saudi Arabia, UAE, Bahrain, Kuwait, Qatar, Oman], North Africa, South Africa, Rest of Middle East and Africa). In addition, analysis comprising market size, Y-O-Y growth & opportunity analysis, market players’ competitive study, investment opportunities, demand for future outlook etc. has also been covered and displayed in the research report.
Growing Prevalence of Liver Cancer and Advancement in Cancer Therapeutics with Emergence of Targeted Therapies to Boost Market Growth
Liver cancer is the sixth most common type of cancer in the world and recorded a number of 905,677 cases in 2020, as per statistics by the International Agency for Research on Cancer (IARC). Growing prevalence of liver cancer over high alcohol consumption, non-alcoholic fatty liver disease, is one of the key factors driving the growth of the market. Moreover, targeted therapy is considered a breakthrough in the field of medical oncology, which determined an improvement in the effectiveness of cancer treatments. Increasing R&D activities and advancement in targeted cancer therapies are also expected to foster the growth of the global liver cancer therapeutics market.
Curious about this latest version of report? Obtain Report Details @ https://www.researchnester.com/reports/liver-cancer-therapeutics-market/4114
However, high cost and side effects related to certain liver cancer therapies, and limitations in treatment options with several therapies in clinical trial stage are expected to restrain the growth of global liver cancer therapeutics market over the forecast period.
This report also provides the existing competitive scenario of some of the key players of the global liver cancer therapeutics market which includes company profiling of Bristol-Myers Squibb Company, Eisai Co., Ltd., Exelixis, Inc., Merck Sharp & Dohme Corp. (Merck & Co., Inc.), Bayer AG, Zymeworks Inc., F. Hoffmann-La Roche Ltd., Celsion Corporation, Eli Lilly and Company, AstraZeneca, among others. The profiling enfolds key information of the companies which encompasses business overview, products and services, key financials and recent news and developments. On the whole, the report depicts detailed overview of the global liver cancer therapeutics market that will help industry consultants, equipment manufacturers, existing players searching for expansion opportunities, new players searching possibilities and other stakeholders to align their market centric strategies according to the ongoing and expected trends in the future.
Ask Industry Experts about this Report @ https://www.researchnester.com/sample-request-4114
0 notes
Text
TOP SELLING BIOLOGICS MARKET - CURRENT MARKET LANDSCAPE
The “Top Selling Biologics Market, report features an extensive study of the current market landscape and future potential of the top selling biologics available for the treatment of a variety of disease indications.
As of today, most of the top revenue generating drugs, across the globe, are biologics. During our biologics market research, we were able to identify 67 top selling biologics (revenues equal to or above USD 500 million in 2020). Top selling biologics market has so far been primarily led by industry players. Majority of industry players have taken a step towards investing their time and resources for the development of biologic therapies. Genentech, Amgen, Janssen Biotech, Sanofi and Novo Nordisk are amongst the major players in this domain. Biogen, AstraZeneca, Merck, Bristol Myers Squibb, GlaxoSmithKline, Alexion Pharmaceuticals, AbbVie and Novartis are other players that have entered the Top Selling Biologics domain in the past.
To request a sample report: https://www.rootsanalysis.com/reports/top-selling-biologics-market/request-sample.html
It is worth highlighting that most of the therapies are monoclonal antibodies capture the highest share (54%) of the top selling biologics market. This can be attributed to the various advantages offered by them, including high reproducibility and specificity. It is also worth mentioning that these therapeutic modalities are being used for many cancer indications. Further, most of the top selling biologics are currently delivered via subcutaneous route of administration. However, most of the top selling biologics are available in the form of vials. This can be attributed to the better sterility assurance and reduced particle presence offered by such packaging formats.
To order customize report: https://www.rootsanalysis.com/reports/top-selling-biologics-market/request-customization.html
It is worth highlighting that most of the therapies are being developed against autoimmune and oncological disorders; Oncological indications targeted by biologics include colorectal cancer (6), Non-Small Cell Lung Cancer (3), Breast Cancer (3), Hepatocellular Carcinoma (3), bladder cancer (3), urothelial carcinoma (3), and melanoma (3).
For more information, please click on the following link:
https://www.rootsanalysis.com/reports/top-selling-biologics-market.html
You may also be interested in the following reports:
1. Antibody Drug Conjugates Market
2. Cell and Gene Therapy CROs Market
3. Bispecific Antibody Therapeutics Market
4. Genome Editing Services Market
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at [email protected]
Contact Information
Roots Analysis Private Limited
Gaurav Chaudhary
+1 (415) 800 3415
#biologics market#biologics market size#biologics market research#biologics market forecast#RootsAnalysis
0 notes
Link
The market analysis report speaks about the growth rate of Hepatocellular Carcinoma Drugs Market till 2028 manufacturing process, key factors driving this market with sales, revenue, and price analysis of top manufacturers of Market, distributors, traders and dealers of Hepatocellular Carcinoma Drugs Market.
0 notes