#Gene Therapy Products Market Trends
Text
In the need for a lovely meta deep dive.
Lovely Writer, it is. (Its a long one, sorry not sorry)
Gene and Nubsib are great but this show as a whole is an amazing pointed look at the problematic layers in the BL world and how different parties contribute to it.
Addressing the lack of consent, the use of coercion, and overall toxic masculine machismo used as a vehicle to get to NSFW scenes. It is refreshing that we see the managers who are notorious for being predatory in these shows actually be supportive and have dimension to their characters. And big ups to Nubsib for refusing to hook up with a drunk Gene.
As a demisexual, I appreciate the conversations around the egregious and gratuitous use of sex to cover for bad scripts and the trend of ignoring the plot for the sake of sex.
The fans' unhinged entitlement and commodification of an actor, the dehumanization and puppetry in fan service, and the hostile industry's romanticism of SA. Possessive parasocial relationships.
The show also does a great job of addressing the subversive caricature assumptions and stereotypes regarding the LGBT+ community within the script and in the shows IRL. The callouts to the hypocrisy and those disrespectful views towards the gay community while also marketing said gayness to straight women. Also, the pressure to come out or stay in the closet is based on how the news can be again commodified.
Oh the coming out scenes in the second half of the show had me clenching everything. It was very tense and you see the subtle hypocrisy in the parents who never consider the possibility of their children being anything but straight.
Homophobia vs Fear of Retaliation: Even though Gene's dad was in love with a man once, he is still not immediately accepting of his son's sexuality. I was cheering for the gumption and boldness when Gene's brother threw that back at his father and reminded him that he was bisexual. It was nice that the father was more supportive and opened up about the fact that his opposition came from fear of his son going through similar trials and pain. Its a nice change from the usual trope of a shitty and homophobic father and open minded.
The continued digs at optics and performativity. Acting, couples, products, fan service, stereotypes, gender norms, media, etc etc.
At its core we have Gene and Nubsib who are dealing with different aspects of all of these issues and the rapid nature of the media, from opposite sides of the lens. We see this a great deal in the Press Conference episode or as I call it, Ghost Ship v Show Ship.
The unreliable narrative across the board is done in a very tongue and cheek way which I appreciate. Even until the end, it's not even hiding what it's doing. Gene was literally the creator of this show that the fans supposedly loved but they were quick to hate him simply for being the person Nubsib actually want to be with.
Though, Aoey irks me and needs more therapy then there are days in the century. I did appreciate the continued dig at the obsessive nature of the fans and how we see that contribute to Aoey's declining mental state . But I refuse to feel more than the requisite level of sympathy for this bottle of chaos especially after the waterworks show on a Live he did to capitalize on the hate being thrown at Gene.
Aoey is what I would describe as a wrecking ball. Not malicious, but destructive all the same. He doesn't spend much time thinking about the consequences of his actions and only focuses on the possibility for attention and love which he craves to the point of obsession. (Like I said, so much therapy). But the show illustrates that flawlessly and shows him alone at the end due to his actions, full of fame but it's hollow.
Mhok was right there but some people craft these self-fulfilling prophecies and force the other shoe to drop instead of accepting love. I'm glad that the show allows Mhok to move on.
Especially at the end where we see Gene and Nubsib forced to lie about their relationship which predates their fame or the show because of how strangers on the internet feel, how it affects the show (within the show) actual ship, and the genuine obsession with the "ghost ship". Toxic behavior all around hurts everyone for the sake of coin.
Nubsib was ready to just give up his career if it meant he could be with the one he loved but Gene, ever the practical one, wasn't willing to allow him to destroy his job. But they thankfully addressed that the temporary nature of putting out media fires was never ending because of the toxic expectations the industry and the fans had.
In short, Lovely Writer did an amazing job of addressing so many of the overarching issues that plague the industry by being a show within a show, a story within a story, and showing the facets of both. And the last scene was a great tie-in, continuing to hint that none and all of it was real at the same time.
I am all for appreciating and loving the acting in a show but I wish more people understood that the people doing the acting are not the characters. And I am not owed their time outside of that. And this show as well as the War of Y are doing good work by continuing to point out the trappings and pitfalls beneath the glitz, sheet masks, snack placements, and all the pretty people.
5 notes
·
View notes
Text
China Biotech Services Holdings Limited: Shareholders Board Members Managers And Company Profile Bmg2152f1014
The space is massive and rising quickly, accelerated by medical trial decentralization. Additionally, it's complicated, and there are more than 15 discrete product markets, with every product market having its personal set of buying for dynamics and different aggressive sets. This creates significant opportunities for disruptive choices and investment for financial traders in each best-in-breed product corporations, and in multiproduct platforms. Several multiproduct platforms acquired investments and/or underwent recapitalizations over the past few years, together with Signant Health (backed by Genstar) and WCG (backed by Arsenal Capital Partners, Leonard Green & Partners, and Novo Holdings). While deal activity was restricted within the area in 2022 outside of Blackstone and CPP Investments’ acquisition of Advarra, we expect much more activity in 2023. For the finance group, this journey from awaiting clinical data to a industrial launch or different vital company life cycle occasions can happen shortly.
According to a McKinsey analysis of pharmaceutical industry information from Evaluate, roughly 400 gene therapies are at present in development; by 2025, they could comprise round 20 percent of recent product launches. The field continues to evolve, with gene enhancing enabling everlasting and precise genetic deletions and ex vivo modifications. From 2019 to 2021, VC corporations invested greater than $52 billion in therapeutic-based biotech companies globally.
In biotech retirement planning boston , we are going to explore how the powerful mixture of AI software program and Sage Intacct can revolutionize accounting workflows, helping Biotech firms streamline their operations, achieve financial insights, and achieve sustainable growth. We are empowering Louisiana innovators to create profitable biotech businesses by providing them with access to personalized commercialization services, premier amenities, and a comprehensive assist network. This doc incorporates common data only and Deloitte just isn't, by means of this document, rendering accounting, business, financial, investment, authorized, tax, or other skilled recommendation or services. This doc is not a substitute for such skilled advice or services, nor should it's used as a basis for any decision or action that may affect your small business. Before making any choice or taking any motion that may affect your small business, you want to consult a professional skilled advisor. Deloitte shall not be responsible for any loss sustained by any person who depends on this doc.
On the opposite hand, some funds may resolve to construct corporations as a result of others are stepping again. Some pharma companies specifically will view this as a compelling time to do extra partnerships with early-stage corporations with interesting science and platforms. That was a fantastic enterprise for them, and they oriented their complete investing practice round this IPO machine. But when this IPO machine is stopped, they want to determine what they’re going to do with all these early-stage investments they made at high valuations.
AI software can empower your biotech startup with real-time financial visibility, offering up-to-date insights into revenue, expenses, money flow, and different key metrics. The 2024 version of Deloitte’s Life Sciences Industry Accounting Guide is here to help deliver readability. As an accounting handbook for pharmaceutical firms and others within the life sciences business, professionals can use the guide all 12 months long to deal with established accounting or reporting points like these and others. They can even learn how to handle new trends in the industry like environmental, social, and governance (ESG) reporting and the potential financial reporting influence of the Inflation Reduction Act (IRA). With a humble start in 2001, we've expanded steadily with very excessive development.
With AI-driven analytics and customizable dashboards, your finance staff can effortlessly monitor financial health, establish tendencies, and make data-driven choices. About Deloitte As used on this doc, “Deloitte” means Deloitte & Touche LLP, a subsidiary of Deloitte LLP. Over the past five years, the Biotechnology within the US industry has grown by 1.5% to succeed in income of $108bn in 2018. In the same timeframe, the variety of businesses has grown by 1.1% and the number of workers has grown by 0.2%.
Establishing a budget is one of the most necessary steps you'll find a way to take throughout this stage. This finances ought to include all your anticipated bills and income for the upcoming fiscal yr. It has become widely accepted to categorize all small drugmakers as belonging to the biotech trade even after they don’t meet the definition of a biotech.
By placing finance on the centre of all enterprise techniques, finance teams can see all of their key metrics on one dashboard and know where they stand in real-time. That also makes it easier for them to report their numbers to stakeholders and traders. Biotech finance groups must spend much less time gathering knowledge and extra time analysing it to inform strategic enterprise selections. Given most finance teams’ measurement and price targets, the one viable option is to automate information capture and consolidation. Securities and advisory services provided via Commonwealth Financial Network®, Member FINRA/SIPC, a Registered Investment Adviser.
Based on analysis and interviews with greater than 100 founders, fintech and banking executives, buyers, and senior ecosystem stakeholders, we've recognized key themes shaping the future of fintechs. To help fintechs capitalize on these themes, we additionally provide a framework for sustainable development, based on an analysis of the methods utilized by long-established public corporations which have weathered earlier financial cycles. The final step in biotech financial planning is to develop a long-term strategy that takes into account their goals and goals. This consists of identifying potential collaboration companions, making a product pipeline that can be developed over time, and figuring out potential acquisition targets. By taking these steps, biotech companies can make sure that they've the sources they want to reach a extremely competitive and ever-changing industry. Biotech CPA startups require substantial investment to fund R&D, medical trials, and regulatory compliance.
#biotech wealth management#biotech financial services#biotech investment advisors#biotech tax planning#biotech estate planning#biotech investment planning#biotech retirement planning#biotech financial services boston#biotech investment advisors boston#biotech tax planning boston#biotech estate planning boston#biotech investment planning boston#biotech retirement planning boston
0 notes
Text
Detailed Report on Continuous Bioprocessing Market | BIS Research
Bioprocessing is a branch of biotechnology that involves the use of living organisms or their components to manufacture products. It encompasses various techniques and processes aimed at utilizing biological systems, such as cells, enzymes, or microorganisms, to produce valuable substances like pharmaceuticals, food ingredients, biofuels, and specialty chemicals.
The Global Bioprocessing Market was valued at $250.1 million in 2023 and is expected to reach $1,639.1 million by 2033, growing at a CAGR of 20.68% between 2023 and 2033.
Continuous Bioprocessing Overview
The continuous bioprocessing market can be defined as the segment of the biopharmaceutical industry that focuses on the production and manufacturing of biologics, vaccines, and pharmaceuticals using continuous and uninterrupted processes, which are opposite to traditional batch processing.
This market includes bioreactors, chromatography systems, consumables, filtration systems and devices, and other related products involved in the streamlined and continuous production of biopharmaceutical products such as monoclonal antibodies, vaccines, cell and gene therapy and other applications, with the aim of improving efficiency, reducing production costs.
Grab the free sample page click here
Scope and Importance of Continuous Bioprocessing Market
Several key aspects highlighting its significance
Production of Pharmaceuticals: Bioprocessing is vital for the production of biopharmaceuticals, including vaccines, antibodies, hormones, and therapeutic proteins.
Biofuels and Renewable Energy: Bioprocessing plays a crucial role in the production of biofuels, such as ethanol, biodiesel, and biohydrogen, providing alternatives to fossil fuels.
Food and Beverage Industry: Bioprocessing is used in the production of various food ingredients, enzymes, flavors, and additives.
Agricultural Biotechnology: Bioprocessing is employed in the development of agricultural bioproducts, including biopesticides, biofertilizers, and plant-based pharmaceuticals.
Sustainability and Green Chemistry: Bioprocessing offers sustainable alternatives to traditional chemical synthesis methods, reducing energy consumption, waste generation, and environmental pollution
Bioprocessing plays a pivotal role in advancing biotechnology, driving economic growth, and addressing global challenges related to health, energy, environment, and food security.
Market Drivers and Trends for Continuous Bioprocessing Market
a) market drivers are as follows
Growing demand for continuous bioprocessing due to flourishing biopharma
Cost reduction with continuous bioprocessing
Increasing adoption of new technologies like single use bioprocessing techniques
b) market trends are as follows
Digitalization of continuous bioprocessing by the biopharmaceutical industry
These drivers and trends are shaping the bioprocessing market landscape, driving innovation, investment, and growth in the biotechnology and biopharmaceutical industries.
Applications for Continuous Bioprocessing Market
Monoclonal Antibodies
Vaccines
Cell and gene therapy
Other applications
And many others
Recent Developments in the Bioprocessing Market
• Waters and Sartorius expanded their partnership to develop integrated analytical tools for downstream biomanufacturing following their successful collaboration in upstream processes.
• Sartorius and Repligen Corporation launched an integrated system with Biostat STR and XCell ATF for upstream process intensification.
Visit our Life Sciences and Biopharma Vertical page for better understanding
Key Players in the market
• 3M
• Bio-Rad Laboratories, Inc.
• Thermo Fisher Scientific, Inc.
• Merck KGaA
• Sartorius AG
• Danaher Corporation
Key Questions Answered
Q What is the estimated global market size for the continuous bioprocessing market?
Q What future trends are expected in the continuous bioprocessing market?
Q What does the supply chain of the continuous bioprocessing market look like?
QWhat does the value chain of the continuous bioprocessing market look like?
Q What is the regulatory framework within the continuous bioprocessing market?
Q What is the patent analysis trend based on country and year in the continuous bioprocessing market?
Q How has the COVID-19 outbreak affected the future trajectory of the continuous bioprocessing market?
Q What are the next frontiers in the continuous bioprocessing market?
Conclusion
In conclusion, the global continuous bioprocessing market is poised for significant growth and innovation in the coming years. With increasing demand for biopharmaceuticals, coupled with the advantages offered by continuous bioprocessing such as enhanced productivity, reduced costs, and improved quality control, the market is witnessing a paradigm shift in biomanufacturing practices.
0 notes
Text
Multiplex Assay Market: Leading Types & APAC’s Dominance

Over the years, there has been a dramatic rise in chronic diseases such as cancer, diabetes, and cardiovascular disorders globally. According to recent studies, around 60% of adults in industrialized nations are diagnosed with at least one chronic condition, sparking significant advancements in diagnostic technologies. Multiplex protein assays have emerged at the forefront, offering a robust solution for the simultaneous detection of multiple biomarkers, which is crucial for diagnosing and managing a range of diseases. As per Triton’s analysis, the Global Multiplex Assay Market is set to reach $8235.49 million by 2032, garnering a CAGR of 8.56% during the 2024-2032 forecast period.
Additionally, multiplex assays are particularly instrumental in tailoring personalized medications enhancing treatment efficacy and patient outcomes. Take warfarin, for instance, a blood clot prevention medication. Its effectiveness varies due to genetic diversity among patients and differences in drug metabolism enzymes. Multiplex protein assays streamline this process by simultaneously analyzing multiple genes, enabling more efficient detection of variations in one test cycle.
Explore in detail about this market in our FREE sample
Multiplex Assay Market: Top Two Types Leading Advancements
Protein-Based Assays
Nucleic Acid-Based Assays
Connect with our experts for a simplified analysis!
Asia-Pacific: A Hotspot for Multiplex Assay Market
The Asia-Pacific multiplex assay market is set to witness the fastest growth at a CAGR of 9.34% during 2024-2032, spearheaded by China.
Countries like China and India are leading this surge, with companies such as Shanghai Luminex, known for their advanced multiplex cytokine assays, playing pivotal roles in the regional market dynamics. The region’s market is buoyed by the increase in local manufacturing of multiplex assay kits and the strategic expansion of international players who are investing in local production facilities to reduce costs and improve accessibility.
The proliferation of Contract Research Organizations (CROs) in China has surged, broadening their services and catalyzing business outsourcing. Chinese firms are leveraging this trend by expanding chemistry services to encompass lead optimization, enzyme and cell assays, ADME, and toxicity studies. This expansion, coupled with competitive costs and innovation, is driving the demand for multiplex assay across China. Simultaneously, the biopharmaceutical sector is diversifying beyond generics, prioritizing personalized medicine, biomarkers, novel therapies, and multiplex assays.
In Conclusion,
As we look towards a future where healthcare is more personalized, predictive, and preventive, multiplex assays stand as a cornerstone technology that will strengthen these advancements. Their ability to provide comprehensive, rapid, and accurate testing solutions across various medical and research applications makes them indispensable in the advancing healthcare industry.
Explore Our Latest Release for the 2024-2032 Market Analysis
FAQs
Q1 What is a multiplex assay?
A multiplex assay is a type of laboratory procedure that allows for the simultaneous measurement of multiple analytes (such as proteins, genes, or biomarkers) in a single assay. It is a tool for diagnostics and research, enabling high-throughput and efficient data collection. Q2 What are the advantages of multiplex assays?
Multiplex assays offer several advantages, including reduced cost, lower sample volume requirements, high throughput, and the ability to provide comprehensive data from a single test, which can enhance diagnostic accuracy and ensure better clinical decisions. Q3 How are multiplex assays contributing to personalized medicine?
Multiplex assays contribute to personalized medicine by allowing for the detailed analysis of an individual’s biomarkers, facilitating tailored treatment strategies that improve patient outcomes and minimize side effects.
#multiplex assay#Lifesciences#Diagnostic & Biotechnology#triton market research#market research reports
0 notes
Text
Regenerative Medicine Market Size, Historical Growth, Analysis, Opportunities and Forecast To 2031

Regenerative medicine has emerged as a beacon of hope in the realm of healthcare, offering novel approaches to address a myriad of medical conditions by harnessing the body's own regenerative capabilities. With a promising trajectory, the regenerative medicine market has witnessed exponential growth in recent years and is poised for even greater expansion in the coming decade. According to recent market analysis, the global regenerative medicine market was estimated at USD 30.01 billion in 2023 and is anticipated to reach USD 105.62 billion by 2031, reflecting a remarkable compound annual growth rate (CAGR) of approximately 17.03% for the forecast period of 2024-2031.
Emerging Trends and Opportunities
Several emerging trends are shaping the landscape of the regenerative medicine market, presenting new opportunities for growth and innovation. One such trend is the increasing adoption of stem cell therapies across a diverse range of medical specialties, including orthopedics, cardiology, and neurology. Stem cells possess the unique ability to differentiate into various cell types, making them invaluable in regenerating damaged tissues and organs.
Furthermore, advances in tissue engineering and biomaterials have paved the way for the development of sophisticated scaffolds and matrices that mimic the native microenvironment of cells, facilitating tissue regeneration with enhanced precision and efficacy. Additionally, the rise of 3D bioprinting technologies holds immense promise for the customization and on-demand fabrication of complex tissue constructs for transplantation and organ replacement.
Moreover, the growing prevalence of chronic diseases, coupled with an aging population, is driving the demand for regenerative therapies that offer long-term solutions and improved quality of life. This demographic shift presents a vast market opportunity for regenerative medicine companies to develop innovative treatments for conditions such as osteoarthritis, cardiovascular disease, and diabetes.
Download Free Sample Report: https://www.snsinsider.com/sample-request/2996
Key Drivers Propelling Growth
Several key drivers are fueling the rapid expansion of the regenerative medicine market. One of the primary drivers is the increasing prevalence of chronic and degenerative diseases, such as cancer, diabetes, and Parkinson's disease. Conventional treatments for these conditions often provide symptomatic relief but fail to address the underlying cause of the disease. Regenerative therapies offer the potential to repair or replace damaged tissues and organs, providing long-lasting benefits for patients.
Moreover, favorable regulatory initiatives and government funding support are bolstering research and development efforts in the field of regenerative medicine. Regulatory agencies are recognizing the potential of regenerative therapies to address unmet medical needs and are streamlining approval processes to accelerate their clinical translation. Additionally, strategic collaborations between academia, industry, and regulatory bodies are fostering innovation and driving the commercialization of regenerative medicine products.
Furthermore, advancements in biotechnology and genetic engineering are expanding the repertoire of tools available for regenerative medicine research and therapy development. Breakthroughs in gene editing technologies, such as CRISPR-Cas9, are enabling precise manipulation of the genome, opening up new avenues for personalized regenerative therapies tailored to individual patient needs.
Challenges and Considerations
Despite the immense potential of regenerative medicine, several challenges and considerations must be addressed to realize its full impact. One of the primary challenges is the complexity and cost associated with developing and manufacturing regenerative therapies. The production of cell-based therapies often requires sophisticated manufacturing processes and infrastructure, leading to high upfront costs and scalability challenges.
Moreover, ensuring the safety and efficacy of regenerative therapies remains a paramount concern. The unpredictable nature of cell behavior and potential risks, such as tumorigenicity and immune rejection, necessitate rigorous preclinical and clinical testing to mitigate adverse outcomes. Additionally, establishing standardized protocols and quality control measures is essential to ensure consistency and reproducibility in the manufacturing of regenerative medicine products.
Furthermore, reimbursement and market access pose significant barriers to the widespread adoption of regenerative therapies. The lack of established reimbursement mechanisms for innovative treatments and the high upfront costs may limit patient access and deter healthcare providers from incorporating regenerative therapies into clinical practice.
Key Takeaways from the Market
In conclusion, the regenerative medicine market is poised for exponential growth, driven by emerging trends, favorable market dynamics, and technological advancements. Stem cell therapies, tissue engineering, and 3D bioprinting are revolutionizing the treatment landscape for a wide range of medical conditions, offering new hope for patients with unmet medical needs.
However, realizing the full potential of regenerative medicine requires overcoming various challenges, including manufacturing complexities, safety concerns, and reimbursement barriers. Strategic collaborations between stakeholders and continued investment in research and development are crucial to address these challenges and unlock the transformative power of regenerative medicine.
As we embark on this journey of innovation and discovery, the regenerative medicine market holds the promise of revolutionizing healthcare and ushering in a new era of regenerative therapies tailored to the individual needs of patients. With concerted efforts and unwavering commitment, the regenerative medicine industry is poised to reshape the future of medicine and improve the lives of millions worldwide.
0 notes
Text
Idiopathic Pulmonary Fibrosis Market Report 2032: Epidemiology Data, Pipeline Therapies, Latest FDA, EMA, PDMA Approvals by DelveInsight | FibroGen, Hoffmann-La Roche Ltd, United Therapeutics, Boehringer Ingelheim, Pliant Therapeutics, Inc., Galecto Biotech, Horizon Therapeutics, CSL Behring, Kadmon Corporation, MediciNova, PureTech, Bristol-Myers Squibb, Nitto Denko Corporation, Vicore Pharma AB
DelveInsight’s “Idiopathic Pulmonary Fibrosis Market Insights, Epidemiology, and Market Forecast-2032″ report offers an in-depth understanding of the Idiopathic Pulmonary Fibrosis, historical and forecasted epidemiology as well as the Idiopathic Pulmonary Fibrosis market trends in the United States, EU4 (Germany, Spain, Italy, France) the United Kingdom and Japan.
To Know in detail about the Idiopathic Pulmonary Fibrosis market outlook, drug uptake, treatment scenario and epidemiology trends, Click here; Idiopathic Pulmonary Fibrosis Market Forecast
Recent Advancements in the Idiopathic Pulmonary Fibrosis Market:
In May 2023, Kinarus Therapeutics disclosed the execution of a strategic convertible loan agreement with ChaoDian (Hangzhou) Investment Management Co., Ltd. ("CDIM"), an investment firm based in Hangzhou City, China, for a CHF 1.5 million investment. This agreement lays the groundwork for discussions regarding the introduction, development, and commercialization of KIN001 for treating Idiopathic Pulmonary Fibrosis (IPF) in China. CDIM was introduced to Kinarus through Great Health Companion Group Ltd (GHCG), a subsidiary of Hakim Unique Group.
In April 2023, AGC Biologics announced the signing of a service agreement with The Jikei University in Japan. This agreement entails AGC Biologics undertaking a technology transfer and feasibility study for a drug product targeting the treatment of Idiopathic Pulmonary Fibrosis (IPF) at the CDMO's Cell and Gene Excellence center in Milan.
In February 2023, Insilico Medicine revealed that the US Food and Drug Administration (FDA) granted Orphan Drug Designation to INS018_055 for Idiopathic Pulmonary Fibrosis (IPF) treatment.
In February 2023, Arrowhead Pharmaceuticals Inc. announced the dosing of the first subjects in a Phase I/IIa clinical trial of ARO-MMP7, the company's investigational RNA interference (RNAi) therapeutic intended to reduce the expression of matrix metalloproteinase 7 (MMP7) as a potential treatment for Idiopathic Pulmonary Fibrosis (IPF).
In February 2023, Daewoong Pharmaceutical of South Korea secured an exclusive licensing agreement with CS Pharmaceuticals for Bersiporocin, a first-in-class PRS inhibitor, in the Greater China region, including mainland China, Hong Kong, Taiwan, and Macau. This agreement enables CSP to license Bersiporocin for Idiopathic Pulmonary Fibrosis (IPF) and potentially other fibrotic indications for a total consideration of up to $336 million, including up to $76 million in upfront and development milestone payments and double-digit royalties on Net Sales.
In January 2023, Insilico Medicine reported positive topline results of safety, tolerability, and pharmacokinetics (PK) from the Phase 1 clinical trial of INS018_055, a potential first-in-class drug discovered by Insilico's end-to-end AI platform for Idiopathic Pulmonary Fibrosis (IPF).
In January 2023, Pliant Therapeutics unveiled 12-week interim data from the 320 mg dose group of INTEGRIS-IPF, a multinational, randomized, double-blind, placebo-controlled Phase 2a clinical trial of bexotegrast (PLN-74809) in patients with Idiopathic Pulmonary Fibrosis (IPF).
In December 2022, Vallon Pharmaceuticals announced the execution of a definitive agreement ("Merger Agreement") wherein GRI Bio would merge with a wholly-owned subsidiary of Vallon in an all-stock transaction ("Merger"). The combined entity is set to concentrate on advancing GRI Bio's innovative pipeline of NKT cell regulators for treating inflammatory, fibrotic, and autoimmune diseases. Post-merger, the combined company is anticipated to operate under the name "GRI Bio, Inc."
In July 2021, FibroGen revealed that FG-3019, their human monoclonal antibody targeting connective tissue growth factor (CTGF), had received Orphan Drug Designation from the U.S. Food and Drug Administration (FDA) for idiopathic pulmonary fibrosis (IPF) treatment.
In June 2021, Redx Pharma announced the initiation of Phase I clinical trials for RXC007, an investigational oral therapy for idiopathic pulmonary fibrosis (IPF) and other fibrotic or scarring-related conditions, with the dosing of the first healthy volunteer.
In May 2021, ImmunoMet Therapeutics disclosed that the U.S. Food and Drug Administration (FDA) had granted fast track status to IM156, a prospective treatment for idiopathic pulmonary fibrosis (IPF).
In March 2021, MyMD Pharmaceuticals reported promising efficacy of their lead candidate, MYMD-1, in targeting the underlying causes of inflammation in idiopathic pulmonary fibrosis (IPF), along with its potential for treating various autoimmune and age-related disorders.
Some of the key facts of the Idiopathic Pulmonary Fibrosis Market Report:
The Idiopathic Pulmonary Fibrosis market size is anticipated to grow with a significant CAGR during the study period (2019-2032).
The Idiopathic Pulmonary Fibrosis market size in seven major markets was USD 3,167 million in 2021
The total Idiopathic Pulmonary Fibrosis diagnosed prevalent cases in the 7MM was 194,878 cases in 2021 which is expected to rise, at a CAGR of 1.1% during the study period (2019–2032).
The expected launch of potential therapies may increase the Idiopathic Pulmonary Fibrosis market size in the coming years, assisted by an increase in the diagnosed prevalent population of Idiopathic Pulmonary Fibrosis.
Upcoming Idiopathic Pulmonary Fibrosis therapies such as Pamrevlumab, PRM-151 (pentraxin-2, RG6354), Tyvaso (treprostinil), BI 1015550, and others has the potential to create a significant positive shift in the Idiopathic Pulmonary Fibrosis market size.
The United States accounts for the largest Idiopathic Pulmonary Fibrosis market size, with approximately USD 2,321 million in 2021 and is expected to increase by 2032 at a Compound Annual Growth Rate (CAGR) of 6.3% for the study period (2019–2032).
The total Idiopathic Pulmonary Fibrosis Market Size in the EU-5 was USD 693 million in 2021, which is anticipated to grow at a CAGR of 7.8%.
Japan accounted for USD 153 million market share in 2021 i.e. 5% of the total Idiopathic Pulmonary Fibrosis Market Size in the 7MM.
Key Idiopathic Pulmonary Fibrosis Companies: FibroGen, Hoffmann-La Roche Ltd, United Therapeutics, Boehringer Ingelheim, Pliant Therapeutics, Inc., Galecto Biotech, Horizon Therapeutics, CSL Behring, Kadmon Corporation, MediciNova, PureTech, Bristol-Myers Squibb, Nitto Denko Corporation, Vicore Pharma AB, and others
Key Idiopathic Pulmonary Fibrosis Therapies: ESBRIET (Pirfenidone), OFEV (Nintedanib), Pamrevlumab, PRM-151 (RG6354), Tyvaso (inhaled treprostinil), and others
The total Idiopathic Pulmonary Fibrosis diagnosed prevalent cases in the 7MM was 194,878 cases in 2021 which is expected to rise, at a CAGR of 1.1% during the study period (2019–2032).
The highest Idiopathic Pulmonary Fibrosis diagnosed prevalent cases was accounted for by the US in 2021, with 94,736 cases in the 7MM, which is expected to show a steep rise soon due to the improvement in diagnostic testing and increasing population.
Among the European countries, Germany had the highest diagnosed prevalent population of IPF with 20,774 cases, followed by the UK with 15,760 cases in 2021. On the other hand, Spain had the lowest diagnosed prevalent population.
In the epidemiology model of DelveInsight, we have considered four age groups for the categorization of IPF i.e. 18–39 years, 40–59 years, 60–79 years, and >80 years. As per our analysis, the highest percentage of diagnosed prevalent cases was observed in age group 60–79, in all the 7MM countries.
As per DelveInsight’s analysis, the males are predominantly affected more highly with IPF than females. In 2021, there were 121,389 males and 73,488 females affected by IPF in the 7MM.
Japan accounted for 21,246 cases of total diagnosed prevalent cases of IPF in 2021 which are anticipated to rise by the end of 2032.
The Idiopathic Pulmonary Fibrosis market is expected to surge due to the disease's increasing prevalence and awareness during the forecast period. Furthermore, launching various multiple-stage Idiopathic Pulmonary Fibrosis pipeline products will significantly revolutionize the Idiopathic Pulmonary Fibrosis market dynamics.
Idiopathic Pulmonary Fibrosis Overview
Idiopathic Pulmonary Fibrosis (IPF) is a chronic lung condition marked by the thickening, stiffening, and scarring (fibrosis) of lung tissue, leading to progressive lung disease and shortness of breath. It is categorized as a type of idiopathic interstitial pneumonia, a group of lung disorders causing similar lung damage of unknown origin, also referred to as diffuse parenchymal lung diseases.
The primary symptom of IPF is breathlessness, particularly evident during physical exertion such as exercise. The exact cause of IPF remains unclear, with both familial and sporadic occurrences observed. Various factors, including immunological, environmental, and genetic elements, are believed to contribute to its development.
Idiopathic Pulmonary Fibrosis Epidemiology
The epidemiology section provides insights into the historical, current, and forecasted epidemiology trends in the seven major countries (7MM) from 2019 to 2032. It helps to recognize the causes of current and forecasted trends by exploring numerous studies and views of key opinion leaders. The epidemiology section also provides a detailed analysis of the diagnosed patient pool and future trends.
Idiopathic Pulmonary Fibrosis Epidemiology Segmentation:
The Idiopathic Pulmonary Fibrosis market report proffers epidemiological analysis for the study period 2019–2032 in the 7MM segmented into:
Total Prevalence of Idiopathic Pulmonary Fibrosis
Prevalent Cases of Idiopathic Pulmonary Fibrosis by severity
Gender-specific Prevalence of Idiopathic Pulmonary Fibrosis
Diagnosed Cases of Episodic and Chronic Idiopathic Pulmonary Fibrosis
Download the report to understand which factors are driving Idiopathic Pulmonary Fibrosis epidemiology trends @ Idiopathic Pulmonary Fibrosis Epidemiology Forecast
Idiopathic Pulmonary Fibrosis Drugs Uptake and Pipeline Development Activities
The drugs uptake section focuses on the rate of uptake of the potential drugs recently launched in the Idiopathic Pulmonary Fibrosis market or expected to get launched during the study period. The analysis covers Idiopathic Pulmonary Fibrosis market uptake by drugs, patient uptake by therapies, and sales of each drug.
Moreover, the therapeutics assessment section helps understand the drugs with the most rapid uptake and the reasons behind the maximal use of the drugs. Additionally, it compares the drugs based on market share.
The report also covers the Idiopathic Pulmonary Fibrosis Pipeline Development Activities. It provides valuable insights about different therapeutic candidates in various stages and the key companies involved in developing targeted therapeutics. It also analyzes recent developments such as collaborations, acquisitions, mergers, licensing patent details, and other information for emerging therapies.
Idiopathic Pulmonary Fibrosis Therapies
ESBRIET (Pirfenidone)
OFEV (Nintedanib)
Pamrevlumab
PRM-151 (RG6354)
Tyvaso (inhaled treprostinil)
Idiopathic Pulmonary Fibrosis Key Companies
FibroGen
Hoffmann-La Roche Ltd
United Therapeutics
Boehringer Ingelheim
Pliant Therapeutics, Inc.
Galecto Biotech
Horizon Therapeutics
CSL Behring
Kadmon Corporation
MediciNova
PureTech
Bristol-Myers Squibb
Nitto Denko Corporation
Vicore Pharma AB
Discover more about therapies set to grab major Idiopathic Pulmonary Fibrosis market share @ Idiopathic Pulmonary Fibrosis Treatment Landscape
Scope of the Idiopathic Pulmonary Fibrosis Market Report
Study Period: 2019–2032
Coverage: 7MM [The United States, EU5 (Germany, France, Italy, Spain, and the United Kingdom), and Japan]
Key Idiopathic Pulmonary Fibrosis Companies: FibroGen, Hoffmann-La Roche Ltd, United Therapeutics, Boehringer Ingelheim, Pliant Therapeutics, Inc., Galecto Biotech, Horizon Therapeutics, CSL Behring, Kadmon Corporation, MediciNova, PureTech, Bristol-Myers Squibb, Nitto Denko Corporation, Vicore Pharma AB, and others
Key Idiopathic Pulmonary Fibrosis Therapies: ESBRIET (Pirfenidone), OFEV (Nintedanib), Pamrevlumab, PRM-151 (RG6354), Tyvaso (inhaled treprostinil), and others
Idiopathic Pulmonary Fibrosis Therapeutic Assessment: Idiopathic Pulmonary Fibrosis current marketed and Idiopathic Pulmonary Fibrosis emerging therapies
Idiopathic Pulmonary Fibrosis Market Dynamics: Idiopathic Pulmonary Fibrosis market drivers and Idiopathic Pulmonary Fibrosis market barriers
Competitive Intelligence Analysis: SWOT analysis, PESTLE analysis, Porter’s five forces, BCG Matrix, Market entry strategies
Idiopathic Pulmonary Fibrosis Unmet Needs, KOL’s views, Analyst’s views, Idiopathic Pulmonary Fibrosis Market Access and Reimbursement
To know more about Idiopathic Pulmonary Fibrosis companies working in the treatment market, visit @ Idiopathic Pulmonary Fibrosis Clinical Trials and Therapeutic Assessment
Table of Contents
1. Idiopathic Pulmonary Fibrosis Market Report Introduction
2. Executive Summary for Idiopathic Pulmonary Fibrosis
3. SWOT analysis of Idiopathic Pulmonary Fibrosis
4. Idiopathic Pulmonary Fibrosis Patient Share (%) Overview at a Glance
5. Idiopathic Pulmonary Fibrosis Market Overview at a Glance
6. Idiopathic Pulmonary Fibrosis Disease Background and Overview
7. Idiopathic Pulmonary Fibrosis Epidemiology and Patient Population
8. Country-Specific Patient Population of Idiopathic Pulmonary Fibrosis
9. Idiopathic Pulmonary Fibrosis Current Treatment and Medical Practices
10. Idiopathic Pulmonary Fibrosis Unmet Needs
11. Idiopathic Pulmonary Fibrosis Emerging Therapies
12. Idiopathic Pulmonary Fibrosis Market Outlook
13. Country-Wise Idiopathic Pulmonary Fibrosis Market Analysis (2019–2032)
14. Idiopathic Pulmonary Fibrosis Market Access and Reimbursement of Therapies
15. Idiopathic Pulmonary Fibrosis Market Drivers
16. Idiopathic Pulmonary Fibrosis Market Barriers
17. Idiopathic Pulmonary Fibrosis Appendix
18. Idiopathic Pulmonary Fibrosis Report Methodology
19. DelveInsight Capabilities
20. Disclaimer
21. About DelveInsight
Latest Reports by DelveInsight
Adalimumab Biosimilar Market | Arbovirus Infection Market | Artificial Pancreas Device System Market | Dental Equipment Market | Gluten Sensitivity Market | Hypothyroidism Market | Inflammatory Bowel Disease Market | Mayus Kinase Jak Inhibitors Market | Mild Dry Eye Market | Mucopolysaccharidosis Market | Oncolytic Virus Cancer Therapy Market | Pyoderma Gangrenosum Market | Transdermal Drug Delivery Devices Market | Intrathecal Pumps Market | Hedgehog Pathway Inhibitors Market | Yellow Fever Market | Laryngeal Cancer Market | Female Infertility Market | Gender Dysphoria Market | Chronic Brain Damage Market | Spain Healthcare Outlook Market | Malignant Fibrous Histiocytoma Market | Asthma Diagnostic Devices Market | Chronic Obstructive Pulmonary Disease Treatment Devices Market | Airway Management Devices Market | Cough Assist Devices Market | Pulse Oximeters Market | Hemodialysis Catheter Devices Market | Chronic Spontaneous Urticaria Market | Gender Dysphoria Market | Germany Healthcare Outlook | Biopsy Devices Pipeline Insight | Bacterial Conjunctivitis Market | Infliximab Biosimilar Insight | Eosinophilic Asthma Market | Cushing Syndrome Market | Functional Dyspepsia Market | Peripherally Inserted Central Catheters (PICC) Devices Market
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Contact Us
Kritika Rehani
+91-9650213330
0 notes
Text
Biopharmaceutical CMO Market Growth Opportunities and Competitive Landscape Report to 2033
Market Definition
A Contract Manufacturing Organization (CMO), also known as a Biopharmaceutical CMO, is a company that provides manufacturing and other services to the pharmaceutical and biotechnology industries. CMOs are an important part of the pharmaceutical supply chain, and they play a vital role in bringing new drugs and therapies to market.
CMOs specialize in the manufacture of active pharmaceutical ingredients (APIs) and finished dosage forms (FDFs). They also provide a range of other services, such as analytical testing, formulation development, and packaging. CMOs are typically large, multinational companies with extensive experience in drug manufacturing.
Market Outlook
The key trends in Biopharmaceutical CMO technology are:
1. The use of biotechnology to develop new drugs and therapies.
2. The use of cell culture and fermentation technologies to produce biopharmaceuticals.
3. The use of monoclonal antibodies and other protein-based drugs.
4. The use of nucleic acid-based drugs and gene therapy.
The biopharmaceutical CMO market is driven by the increasing demand for biopharmaceuticals, the need for specialized manufacturing facilities, and the increasing number of biopharmaceutical companies. The biopharmaceutical industry is growing at a rapid pace, and the number of biopharmaceutical companies is increasing. This is resulting in an increased demand for CMOs. CMOs are specialized manufacturing facilities that are required for the production of biopharmaceuticals. They are required to meet the stringent quality standards set by the FDA. The increasing number of biopharmaceutical companies is resulting in an increased demand for CMOs.
The biopharmaceutical CMO market is facing a number of key restraints and challenges. Firstly, the market is highly competitive and there are a large number of players operating in the space. This makes it difficult for new entrants to gain a foothold in the market. Secondly, the market is capital intensive and requires significant investment in research and development. This is a major barrier for small and medium sized companies. Thirdly, the regulatory environment is constantly changing and this makes it difficult for companies to keep up with the latest regulations. Finally, the market is reliant on a small number of key customers and this makes it difficult to diversify revenue streams.
To Know More: https://www.globalinsightservices.com/reports/biopharmaceutical-cmo-market/
Research Objectives
Estimates and forecast the overall market size for the total market, across product, service type, type, end-user, and region
Detailed information and key takeaways on qualitative and quantitative trends, dynamics, business framework, competitive landscape, and company profiling
Identify factors influencing market growth and challenges, opportunities, drivers and restraints
Identify factors that could limit company participation in identified international markets to help properly calibrate market share expectations and growth rates
Trace and evaluate key development strategies like acquisitions, product launches, mergers, collaborations, business expansions, agreements, partnerships, and R&D activities
Thoroughly analyze smaller market segments strategically, focusing on their potential, individual patterns of growth, and impact on the overall market
To thoroughly outline the competitive landscape within the market, including an assessment of business and corporate strategies, aimed at monitoring and dissecting competitive advancements.
Identify the primary market participants, based on their business objectives, regional footprint, product offerings, and strategic initiatives
Request Sample: https://www.globalinsightservices.com/request-sample/GIS21593/
Market Segmentation
The biopharmaceutical CMO market report is bifurcated on the basis of product, source, service, and region. On the basis of product, it is segmented into biologics and biosimilars. Based on source, it is analyzed across mammalian and non-mammalian. By service, it is categorized into contract manufacturing, process development, packaging, and others. Region-wise, it is studied across North America, Europe, Asia-Pacific, and rest of the World.
Request Customization@ https://www.globalinsightservices.com/request-customization/GIS21593/
Major Players
The biopharmaceutical CMO market report includes players such as Toyobo Co., Ltd., Samsung Biologics, Patheon, Lonza AG, WuXi Biologics, AbbVie Inc., Binex Co., Ltd., JRS Pharma, Biomeva GmbH, and ProBioGen AG.
Request Discounted Pricing@ https://www.globalinsightservices.com/request-special-pricing/GIS21593/
Research Scope
Scope – Highlights, Trends, Insights. Attractiveness, Forecast
Market Sizing – Product Type, End User, Offering Type, Technology, Region, Country, Others
Market Dynamics – Market Segmentation, Demand and Supply, Bargaining Power of Buyers and Sellers, Drivers, Restraints, Opportunities, Threat Analysis, Impact Analysis, Porters 5 Forces, Ansoff Analysis, Supply Chain
Business Framework – Case Studies, Regulatory Landscape, Pricing, Policies and Regulations, New Product Launches. M&As, Recent Developments
Competitive Landscape – Market Share Analysis, Market Leaders, Emerging Players, Vendor Benchmarking, Developmental Strategy Benchmarking, PESTLE Analysis, Value Chain Analysis
Company Profiles – Overview, Business Segments, Business Performance, Product Offering, Key Developmental Strategies, SWOT Analysis
Buy your copy here: https://www.globalinsightservices.com/checkout/single_user/GIS21593/
With Global Insight Services, you receive:
10-year forecast to help you make strategic decisions
In-depth segmentation which can be customized as per your requirements
Free consultation with lead analyst of the report
Infographic excel data pack, easy to analyze big data
Robust and transparent research methodology
Unmatched data quality and after sales service
Contact Us:
Global Insight Services LLC
16192, Coastal Highway, Lewes DE 19958
E-mail: [email protected]
Phone: +1-833-761-1700
Website: https://www.globalinsightservices.com/
0 notes
Text
Advancements in Pharmaceutical Development and Manufacturing: Shaping the Future of Healthcare
In the dynamic landscape of healthcare, few industries have undergone as significant transformations as pharmaceuticals. The realm of pharmaceutical development and manufacturing stands at the forefront of innovation, driving the creation of life-saving medications and treatments that improve global health outcomes. Recent advancements in this field have not only accelerated the process of bringing new drugs to market but have also enhanced their efficacy, safety, and accessibility. In this blog post, we'll delve into some of the noteworthy developments shaping the pharmaceutical industry today.
Precision Medicine and Personalized Therapies
One of the most promising trends in pharmaceutical development is the rise of precision medicine. Unlike traditional approaches that offer generalized treatments, precision medicine tailors medical interventions to the individual characteristics of each patient. This approach takes into account genetic makeup, lifestyle factors, and environmental influences to deliver targeted therapies with higher efficacy and fewer side effects.
Technological breakthroughs, particularly in genomics and biotechnology, have facilitated the advancement of personalized medicine. The ability to sequence an individual's genome quickly and affordably has unlocked valuable insights into the genetic basis of diseases, enabling researchers to identify novel drug targets and develop treatments tailored to specific patient populations.
Biopharmaceuticals and Gene Therapies
Biopharmaceuticals, including monoclonal antibodies, therapeutic proteins, and vaccines, have emerged as vital components of modern medicine. Unlike traditional small-molecule drugs, biopharmaceuticals are derived from living organisms and offer unique therapeutic benefits. They are highly targeted, exhibit lower toxicity, and often provide more effective treatment options for complex diseases such as cancer, autoimmune disorders, and infectious diseases.
Furthermore, gene therapies represent a revolutionary approach to treating genetic disorders by correcting or replacing defective genes. Recent breakthroughs in gene editing technologies, such as CRISPR-Cas9, have paved the way for unprecedented precision and efficiency in modifying genetic material, opening new avenues for the treatment of previously incurable conditions.
Digital Transformation and Data Analytics
The pharmaceutical industry is undergoing a digital revolution driven by advancements in data analytics, artificial intelligence (AI), and machine learning. These technologies are being leveraged across the drug development lifecycle to streamline processes, enhance decision-making, and accelerate innovation.
By harnessing vast amounts of data from clinical trials, electronic health records, and real-world evidence, researchers can gain deeper insights into disease mechanisms, drug responses, and patient outcomes. This data-driven approach enables pharmaceutical companies to identify promising drug candidates more efficiently, optimize clinical trial designs, and predict potential safety issues earlier in the development process.
Supply Chain Resilience and Sustainability
Recent global events, such as the COVID-19 pandemic, have underscored the importance of building resilient and sustainable supply chains in the pharmaceutical industry. Disruptions in the supply of raw materials, active pharmaceutical ingredients (APIs), and finished products have highlighted vulnerabilities in the traditional supply chain model and spurred efforts to diversify sourcing, enhance manufacturing capabilities, and improve inventory management.
Moreover, there is growing recognition of the need to adopt environmentally sustainable practices across the pharmaceutical value chain. From reducing energy consumption and greenhouse gas emissions to minimizing waste and pollution, pharmaceutical companies are increasingly embracing sustainability as a core business imperative. This entails investing in green technologies, optimizing manufacturing processes, and collaborating with suppliers to achieve greater sustainability and minimize environmental impact.
Conclusion
The pharmaceutical industry is in the midst of a transformative period characterized by unprecedented innovation, technological advancement, and global collaboration. From precision medicine and biopharmaceuticals to digital transformation and sustainability, recent developments are reshaping the future of healthcare and paving the way for more personalized, effective, and sustainable treatments.
As the industry continues to evolve, pharmaceutical companies must remain agile, adaptable, and committed to driving positive change in the pursuit of better health outcomes for all. By leveraging cutting-edge technologies, fostering innovation, and embracing sustainable practices, the pharmaceutical sector has the potential to address some of the most pressing health challenges facing society today and improve the lives of millions around the world.
0 notes
Text
Preserving Medicinal Integrity: Pharmaceutical Packaging Market Trends
In the world of pharmaceuticals, the role of packaging extends far beyond mere containment; it serves as a crucial guardian of medicinal integrity, ensuring the safety, efficacy, and stability of drugs from production to consumption. The pharmaceutical packaging market is a dynamic sector, constantly evolving to meet the stringent requirements of the pharmaceutical industry while adapting to emerging trends and challenges. From protecting against contamination and tampering to enhancing patient convenience and compliance, pharmaceutical packaging plays a vital role in safeguarding public health and well-being.
At the heart of the pharmaceutical packaging market lies the need to maintain the integrity and potency of medications throughout their lifecycle. Pharmaceuticals are highly sensitive compounds that can degrade when exposed to light, moisture, oxygen, or temperature fluctuations. Packaging materials and designs must therefore be carefully selected to provide an effective barrier against environmental factors that could compromise drug stability and efficacy. Additionally, packaging must prevent microbial contamination and protect against tampering to ensure the safety and purity of medications.
Moreover, the pharmaceutical packaging market is driven by regulatory requirements and quality standards that govern the design, production, and distribution of pharmaceutical packaging materials. Regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) set strict guidelines for pharmaceutical packaging to ensure compliance with Good Manufacturing Practices (GMP) and other quality assurance standards. Packaging manufacturers must adhere to these regulations and undergo rigorous testing and validation to ensure the safety and efficacy of their products.
Request the sample copy of report @ https://www.globalinsightservices.com/request-sample/GIS20240
Additionally, advancements in technology and materials are driving innovation in the pharmaceutical packaging market, leading to the development of novel packaging solutions that offer enhanced protection, functionality, and patient convenience. For example, barrier films and coatings are being used to improve the stability of sensitive drugs, while smart packaging technologies, such as RFID tags and NFC sensors, enable real-time monitoring of medication usage and adherence. Furthermore, advances in packaging automation and robotics are streamlining production processes and improving efficiency in pharmaceutical packaging operations.
Furthermore, the pharmaceutical packaging market is responding to emerging trends and challenges in the healthcare industry, such as the rise of biologics, personalized medicine, and telemedicine. Biologics, including vaccines, antibodies, and gene therapies, present unique packaging requirements due to their sensitivity to temperature and handling. Personalized medicine, which involves tailoring treatment regimens to individual patients, requires flexible and customizable packaging solutions. Additionally, the shift towards telemedicine and remote healthcare delivery has increased demand for home-use packaging formats and patient-friendly designs.
Despite the opportunities for growth, the pharmaceutical packaging market also faces challenges, including cost pressures, sustainability concerns, and supply chain disruptions. Pharmaceutical companies are under pressure to reduce packaging costs while maintaining product quality and compliance with regulatory requirements. Additionally, there is growing awareness of the environmental impact of pharmaceutical packaging, leading to increased demand for sustainable packaging materials and recyclable solutions. Furthermore, supply chain disruptions, such as raw material shortages and transportation delays, can impact packaging availability and lead to product shortages.
In conclusion, the pharmaceutical packaging market is a critical component of the pharmaceutical industry, ensuring the safety, efficacy, and integrity of medications from production to patient use. With its focus on quality, compliance, and innovation, the pharmaceutical packaging sector plays a vital role in safeguarding public health and well-being. As the healthcare landscape continues to evolve and new challenges emerge, the pharmaceutical packaging market will remain at the forefront of efforts to preserve medicinal integrity and enhance patient outcomes.
0 notes
Text
Protein Expression Market Outlines, Future Trends, Insight And Quality Analysis

Protein Expression is a powerful technique used to produce proteins in large quantities by introducing the gene encoding the protein of interest into a host organism, typically bacteria, yeast, insect cells, or mammalian cells
The Recombinant Protein Expression was valued at $2,393.0 million in 2023 and is expected to reach $6,963.6 million by 2033, growing at a CAGR of 11.27% between 2023 and 2033
Gene Expression Analysis Overview
Selection of Expression System: The choice of expression system depends on factors such as the size and complexity of the protein, desired post-translational modifications, and downstream applications.
Common expression systems include bacterial (e.g., Escherichia coli), yeast (e.g., Saccharomyces cerevisiae), insect cells (e.g., Sf9 cells), and mammalian cells (e.g., Chinese hamster ovary cells).
Cloning of the Gene: The gene encoding the protein of interest is isolated and cloned into an expression vector. The vector contains regulatory elements such as promoters and enhancers that drive gene expression in the host organism.
Transformation or Transfection: The recombinant expression vector is introduced into the host organism by transformation (in bacteria and yeast) or transfection (in insect cells and mammalian cells).
Protein Production: The host cells are cultured under optimized conditions to produce the recombinant protein.
Protein Purification: After protein expression, the recombinant protein is purified from the host cell lysate or culture supernatant.
Market Segmentation
Segmentation 1: By Application
Segmentation 2: By End User
Segmentation 3: By Product
Segmentation 4: By Expression System
Segmentation 5: By Region
Protein expression in North America is a dynamic and crucial field with a significant impact across industries.
The region, especially North America, is a global leader in biopharmaceuticals, relying extensively on protein expression for producing biologics, including monoclonal antibodies and vaccines.
North America holds the largest share of the protein expression market
Download our sample page now click here !
Application for Recombinant Protein Expression Market
Drug Discovery
Structural Biology
Disease Modelling
Enzyme Production
Vaccines Development
Therapeutic Proteins
Immunoassays
Key Market Players
Agilent Technologies, Inc.
Bio-Rad Laboratories, Inc.
Charles River Laboratories International, Inc.
Danaher Corporation (Abcam plc.)
GenCefe Co., Ltd.
Genscript Biotech Corporation
And many others
Market Dynamics
Market Drivers
Increasing Demand for Protein Biologics Creating the Need for Protein Expression
Market Restraints
Long and Complicated Regulatory Timelines and Approvals of Recombinant Proteins and Biologics
Market Opportunities
Rising Awareness of Proteomics in Emerging Countries
Visit our Life Sciences & Biopharma page for better understanding
Key factors contributing to the growth of the recombinant protein expression market
Expanding applications of recombinant proteins in drug discovery, biomanufacturing, and diagnostic assays
Rising prevalence of chronic diseases and the need for innovative therapies.
Recent Developments in the Recombinant Protein Expression Market
In January 2024, Evosep, a leader in sample preparation for mass spectrometry-based proteomics, partnered with Thermo Fisher Scientific Inc., a global scientific leader, to advance clinical proteomics research. This collaboration would combine Evosep's sample separation technology with Thermo Fisher Scientific Inc.'s mass spectrometry instruments, enhancing proteomics research capabilities.release would support pharmaceutical and biotechnology companies engaged in the manufacturing of therapeutic proteins, with the goal of improving product quality and expediting time-to-market.
Key Questions Answered
Q What is the estimated global market size for the protein expression market?
Q What are the future trends expected in the protein expression market?
Q What does the supply chain and value chain of the protein expression market look like?
Q What is the regulatory framework of the protein expression market?
Q How has the COVID-19 outbreak affected the future trajectory of the protein expression market?
Q What are the market entry barriers and opportunities in the protein expression market?
Q What are the major market drivers, challenges, and opportunities of the protein expression market?
Q How is each segment of the protein expression market expected to grow during the forecast period, and what is the anticipated revenue generated by each of the segments by the end of 2033?
Q What is the growth potential of the global protein expression market in North America, Europe, Asia-Pacific, Latin America, and Rest-of-the-World, and what are the driving and challenging factors of the market in each of these regions?
Q Who are the leading players with significant offerings in the protein expression market, and what is the current market dominance for each of these leading players? Who are the next frontiers in the protein expression market?
Conclusion
In conclusion, theProtein expression market continues to thrive and evolve as a vital component of numerous industries, including biotechnology, pharmaceuticals, agriculture, and research
0 notes
Text
Gene Synthesis Market: Driving Factors And Global Trends
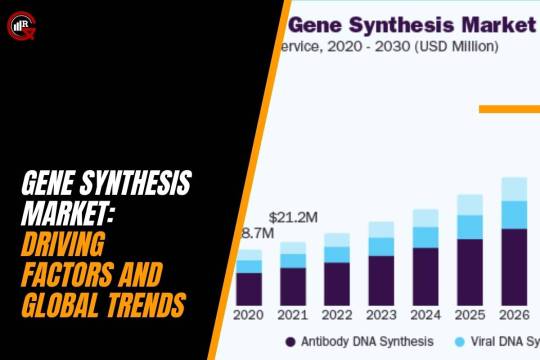
(Source-grandviewresearch)
Gene Synthesis Market, a pivotal technique in synthetic biology, continues to shape the biotechnology landscape, with the United States emerging as a leader in North America. This synthetic approach involves engineering artificial genes in laboratory settings, streamlining the conversion from genetic sequences into physical constructs with precision. Unlike natural gene synthesis reliant on DNA within living organisms, artificial gene synthesis bypasses this constraint, enabling chemically produced genes by skilled professionals.
The United States’ dominance in this market is underpinned by various factors, including the prevalence of genetic and chronic disorders, notably cancer, an ageing population, escalating demand for personalized medicine, and supportive governmental initiatives. Key market players’ presence alongside substantial scientific research investments further fortifies the country’s position. Notably, in the fiscal year 2021, the National Cancer Institute received a dedicated budget of USD 6.56 billion, emphasizing the nation’s commitment to advancing medical knowledge.
Regional Stance:
North America emerges as the largest market for gene synthesis, driven by intensified research and development efforts, breakthrough innovations, expanding gene therapy applications, and the growing burden of chronic ailments. Meanwhile, the Asia Pacific region is poised to become the second-largest market, buoyed by burgeoning demands from the healthcare sector and an upsurge in gene therapy adoption. Additionally, gene synthesis markets are experiencing growth across other continents, including Europe, Latin America, the Middle East, and Africa.
Factors Driving Growth:
Advanced technologies underpinning gene synthesis, leveraging biological synthetic processes, witness widespread adoption, propelling market expansion. DNA-based technologies geared toward protein production and function enhancement serve as pivotal growth drivers. Moreover, the relentless pursuit of research and technology for novel gene synthesis, particularly in long-term therapeutic avenues like targeted and immunotherapy, fuels market growth. The escalating prevalence of chronic disorders, notably oncological ailments, drives demand for innovative therapies, further catalyzing market growth.
Gene synthesis Market Global Trends:
On a global scale, demographic shifts such as ageing populations and the soaring incidence of chronic diseases exert continuous pressure on healthcare systems, stimulating gene synthesis market growth. The advent of personalized medicine holds promise for elevated patient care, enhanced safety, and reduced healthcare costs. For instance, data from the International Diabetes Federation underscore the urgency for healthcare advancements, with projections indicating a substantial increase in diabetes cases by 2045. Such statistics underscore the critical role of innovative healthcare solutions, including gene synthesis, in addressing pressing global health challenges.
0 notes
Text
The Rise of Tech-Driven Therapies: Reshaping the Global Biologics Market
The global biologics market is projected to witness significant growth, reaching a staggering US$817.48 billion by 2032. This substantial increase reflects a Compound Annual Growth Rate (CAGR) of 8.5%, building upon a market valuation of approximately US$335.43 billion in 2021.
Biologics represent a revolutionary class of drugs derived from living organisms or their biological processes. These complex medications, including therapeutic proteins, vaccines, and gene therapies, offer groundbreaking treatment options for various chronic and debilitating diseases.
Obtain Your Sample Report Copy: https://www.futuremarketinsights.com/reports/sample/rep-gb-1249
Market Growth Driven by Multiple Factors:
Several key trends are propelling the biologics market forward:
Rising Chronic Disease Burden: The increasing prevalence of chronic diseases like cancer, autoimmune disorders, and diabetes is driving the demand for effective treatment options, fueling the biologics market.
Enhanced Diagnostics: Advancements in diagnostic tools lead to earlier and more accurate disease detection, enabling early intervention with biologics.
Government Support: Growing government initiatives focused on public health and research & development (R&D) are fostering the development and accessibility of novel biologics.
Technological Advancements: Continuous breakthroughs in biotechnology techniques are leading to the creation of more targeted and effective biologics.
Key Players:
Novartis AG
Pfizer Inc
Dickinson & Company
Smith’s Medicals
Roche Diagnostics
AstraZeneca
Bayer AG
GSK Biologicals
Samsung BioLogics
Merck & Co., Inc.
Eli Lilly and Company
Hoffmann-La Roche Ltd
AstraZeneca.
Key Segments Covered in the Biologics Industry Survey
By Product:
Monoclonal Antibodies
Recombinant Hormones/Proteins
Vaccines
Cellular Based Biologics
Gene-Based Biologics
Therapeutic Enzymes
Others
By Application:
Infectious Diseases
Cancer
Autoimmune Diseases
Rare Diseases
Others
By Drug Classification:
Branded Drugs
Generic Drugs
By Mode of Purchase:
Prescription Drugs
Over-The-Counter (OTC) Drugs
By Distribution Channel:
Hospital Pharmacies
Retail Pharmacies
Online Pharmacies
By Region:
North America
Latin America
Europe
East Asia
South Asia
Oceania
Middle East and Africa
0 notes
Text
MicroRNA Market by Manufacturers, Regions, Type and Application
The global microRNA market has been experiencing substantial growth and is poised for further expansion in the coming years. According to recent market research, the microRNA market size was valued at USD 1.58 billion in 2022 and is projected to reach USD 4.03 billion by 2030, growing at a compound annual growth rate (CAGR) of 12.44% during the forecast period from 2023 to 2030. This remarkable growth can be attributed to several key factors driving the demand for microRNA technologies and therapies.
Emerging Trends and Opportunities
1. Therapeutic Applications
MicroRNAs have gained significant attention in the field of therapeutics due to their regulatory functions in gene expression. Researchers and pharmaceutical companies are exploring the potential of microRNAs as novel targets for the treatment of various diseases, including cancer, cardiovascular diseases, neurological disorders, and metabolic disorders.
2. Diagnostic Biomarkers
MicroRNAs serve as promising biomarkers for disease diagnosis and prognosis. The growing interest in precision medicine and personalized healthcare has led to the development of microRNA-based diagnostic tests for early disease detection and monitoring treatment response. The demand for accurate and non-invasive diagnostic tools is expected to drive the adoption of microRNA diagnostics.
3. Research Tools and Services
The demand for microRNA research tools and services is on the rise, fueled by increasing research activities in molecular biology, genetics, and epigenetics. Companies offering microRNA isolation kits, detection assays, and bioinformatics tools are witnessing growing demand from academic research institutions, biotechnology firms, and pharmaceutical companies.
4. Therapeutic Delivery Systems
Efficient delivery of microRNA therapeutics remains a challenge in the field. Researchers are exploring innovative delivery systems, including lipid nanoparticles, viral vectors, exosomes, and polymer-based carriers, to enhance the stability, specificity, and targeting efficiency of microRNA-based drugs. Advancements in delivery technologies are expected to unlock new opportunities in microRNA therapeutics.
Get Free PDF Sample Copy of Report: https://www.snsinsider.com/sample-request/3143
Key Drivers Propelling Growth
1. Increasing Prevalence of Chronic Diseases
The rising incidence of chronic diseases, such as cancer, cardiovascular disorders, and diabetes, is driving the demand for innovative therapeutic and diagnostic solutions. MicroRNAs play crucial roles in disease pathogenesis and progression, making them attractive targets for therapeutic intervention and biomarker development.
2. Advancements in Omics Technologies
Technological advancements in genomics, transcriptomics, and proteomics have facilitated the discovery and characterization of microRNAs involved in various biological processes and disease pathways. High-throughput sequencing, microarray analysis, and bioinformatics tools have accelerated microRNA research and contributed to the identification of potential therapeutic targets and biomarkers.
3. Growing Investments in Biotechnology and Pharma
Increased investment in biotechnology and pharmaceutical research and development (R&D) is fueling innovation in the microRNA market. Biotech startups and pharmaceutical companies are actively investing in microRNA-based therapeutics, diagnostics, and research tools, driving collaborations, partnerships, and licensing agreements in the industry.
4. Favorable Regulatory Environment
The regulatory landscape for microRNA-based products is evolving, with regulatory agencies providing guidelines for the development and approval of microRNA therapeutics and diagnostics. Regulatory initiatives aimed at expediting the review and approval process for innovative therapies are expected to facilitate market entry and commercialization of microRNA products.
Challenges and Considerations
1. Delivery Challenges
Efficient delivery of microRNA molecules to target cells and tissues remains a major hurdle in therapeutic development. Overcoming barriers such as stability, off-target effects, and immunogenicity poses significant challenges for researchers and drug developers.
2. Biomarker Validation
Validation of microRNA biomarkers for clinical use requires robust clinical studies and validation assays to establish their sensitivity, specificity, and predictive value. Standardization of experimental protocols and collaboration among academia, industry, and regulatory agencies are essential for advancing microRNA diagnostics.
3. Intellectual Property Rights
The competitive landscape of the microRNA market is shaped by intellectual property rights and patents covering microRNA sequences, therapeutic targets, and delivery technologies. Intellectual property disputes and licensing agreements may impact market dynamics and access to proprietary technologies.
4. Ethical and Regulatory Considerations
Ethical considerations surrounding the use of microRNA technologies in human subjects and the potential implications of genetic manipulation require careful evaluation. Regulatory compliance with data privacy, informed consent, and ethical standards is paramount in microRNA research and clinical applications.
Key Takeaways from the Market
The microRNA market is poised for significant growth, driven by therapeutic advancements, diagnostic innovations, and research breakthroughs. Key takeaways from the market include:
Expanding Therapeutic Landscape: MicroRNA-based therapeutics hold promise for addressing unmet medical needs in various disease areas, including oncology, cardiovascular diseases, and neurodegenerative disorders.
Diverse Applications: MicroRNAs are versatile molecules with applications ranging from basic research to clinical diagnostics and therapeutic development, offering diverse opportunities for market players.
Collaborative Ecosystem: Collaboration and partnerships among academia, industry, and regulatory agencies are essential for advancing microRNA research and translating scientific discoveries into clinical applications.
Investment Opportunities: The growing investment in microRNA technologies and the expanding market landscape present lucrative opportunities for investors, startups, and established companies alike.
In conclusion, the microRNA market is witnessing rapid growth and innovation, driven by emerging trends, technological advancements, and increasing demand for precision medicine solutions. Overcoming challenges related to delivery, biomarker validation, and regulatory compliance will be critical for realizing the full potential of microRNA-based therapies and diagnostics in improving patient outcomes and advancing healthcare.
Buy This Exclusive Report: https://www.snsinsider.com/checkout/3143
0 notes
Text
Understanding the Dynamics of the Dry Age-related Macular Degeneration Market: Drivers, Barriers, and Future Outlook
Dry age-related macular degeneration (AMD) is a chronic eye condition characterized by the gradual deterioration of the macula, a small area near the center of the retina responsible for sharp, central vision. Dry AMD is the most common form of AMD, accounting for approximately 85-90% of all cases. Unlike wet AMD, which involves abnormal blood vessel growth beneath the macula, dry AMD typically progresses more slowly and is characterized by the accumulation of yellow deposits called drusen in the macula.
Dry Age-related Macular Degeneration Market Drivers
Aging Population: The aging population is a significant driver of the dry AMD market, as AMD primarily affects individuals over the age of 50. With demographic trends indicating a growing proportion of elderly individuals worldwide, the prevalence of dry AMD is expected to increase, driving demand for diagnostic services, treatments, and supportive care products.
Rising Disease Burden: Dry AMD is a leading cause of vision loss and blindness in older adults, contributing to a substantial disease burden and socioeconomic impact. As the prevalence of dry AMD continues to rise, particularly in developed countries with aging populations, there is an increasing need for effective management strategies to prevent disease progression and preserve vision.
Advancements in Diagnostic Technologies: Technological advancements in imaging modalities, such as optical coherence tomography (OCT), fundus autofluorescence (FAF), and adaptive optics imaging, have improved the early detection and monitoring of dry AMD. These non-invasive imaging techniques enable more accurate assessment of retinal changes, drusen morphology, and disease progression, facilitating timely intervention and personalized treatment approaches.
Research and Innovation: Ongoing research efforts aimed at elucidating the pathogenesis of dry AMD, identifying novel therapeutic targets, and developing innovative treatment modalities drive innovation in the dry AMD market. Research areas of interest include anti-inflammatory agents, neuroprotective compounds, stem cell therapy, gene therapy, and drug delivery systems designed to target specific pathways implicated in AMD pathophysiology.
Clinical Trial Activity: The increasing prevalence of dry AMD and the need for effective treatment options have led to a surge in clinical trial activity focused on evaluating investigational therapies for dry AMD. Pharmaceutical companies, biotechnology firms, academic institutions, and government agencies are conducting clinical trials to assess the safety, efficacy, and tolerability of novel drugs, biologics, gene therapies, and cell-based interventions for dry AMD.
Regulatory Support and Incentives: Regulatory agencies provide support and incentives to expedite the development and approval of new treatments for dry AMD. Designations such as orphan drug status, fast track designation, breakthrough therapy designation, and priority review designation streamline the regulatory review process and accelerate market access for promising therapies targeting unmet medical needs in dry AMD.
Patient Advocacy and Awareness: Patient advocacy organizations and support groups play a crucial role in raising awareness about dry AMD, educating patients and caregivers, and advocating for improved access to treatment and supportive care services. Increased awareness of the importance of early detection, regular eye exams, and adherence to treatment regimens promotes proactive management of dry AMD and enhances patient outcomes.
Healthcare Infrastructure and Access to Care: Access to comprehensive eye care services, including retinal specialists, low vision rehabilitation programs, and low vision aids, is essential for effectively managing dry AMD and optimizing visual function. Investments in healthcare infrastructure, telemedicine platforms, and community-based outreach programs expand access to eye care services, particularly in underserved areas with limited access to specialty care.
Market Competition and Collaboration: Competition among pharmaceutical companies, biotechnology firms, and medical device manufacturers drives innovation and investment in the dry AMD market. Collaborations, partnerships, and licensing agreements between industry players facilitate the development and commercialization of novel therapies, diagnostic technologies, and supportive care products, enhancing market competitiveness and diversifying treatment options for patients.
Reimbursement Landscape: Reimbursement policies, coverage decisions, and pricing strategies influence market dynamics and access to dry AMD treatments. Payer reimbursement for diagnostic tests, treatments, and supportive care services impacts patient access and affordability, driving market adoption and utilization of approved therapies.
Dry Age-related Macular Degeneration Market Barriers
Despite the significant progress in understanding dry age-related macular degeneration (AMD) and developing treatments, several barriers impede the effective management and commercialization of therapies in the dry AMD market. Here are some of the key barriers:
Limited Treatment Options: Compared to wet AMD, there are fewer approved treatment options for dry AMD. Currently, there is no cure for dry AMD, and available treatments mainly focus on slowing disease progression rather than reversing vision loss. The lack of effective pharmacological interventions targeting the underlying mechanisms of dry AMD represents a significant barrier to addressing unmet medical needs in this patient population.
Complexity of Disease Pathophysiology: Dry AMD is a multifactorial disease with complex pathophysiology involving interactions between genetic, environmental, and lifestyle factors. The heterogeneous nature of dry AMD presents challenges for developing targeted therapies that address the diverse underlying mechanisms contributing to disease progression. Understanding the underlying pathophysiological processes and identifying effective therapeutic targets require further research and preclinical validation.
Difficulty in Early Detection and Diagnosis: Early detection and diagnosis of dry AMD are crucial for implementing timely interventions and preserving vision. However, early-stage dry AMD may be asymptomatic or present with subtle visual changes that are challenging to detect using conventional screening methods. Limited access to advanced diagnostic technologies, such as optical coherence tomography (OCT) and fundus autofluorescence (FAF), in primary care settings may delay diagnosis and initiation of treatment.
Lack of Biomarkers for Disease Progression: Biomarkers that reliably predict disease progression and treatment response in dry AMD are currently lacking. The absence of validated biomarkers hinders risk stratification, patient selection for clinical trials, and monitoring of treatment efficacy. Biomarker discovery efforts focusing on identifying molecular, genetic, and imaging-based markers associated with disease progression and treatment response are ongoing but face challenges in reproducibility and validation.
High Development Costs and Long Regulatory Pathways: Developing novel therapies for dry AMD involves substantial investment in research and development, preclinical studies, clinical trials, and regulatory approval processes. The high development costs and lengthy regulatory pathways associated with bringing new drugs to market pose financial barriers for small biotechnology firms and academic researchers. Additionally, uncertainties regarding regulatory requirements and endpoints for clinical trials in dry AMD may prolong the development timeline and increase the risk of clinical trial failure.
Limited Patient Access to Care and Treatment: Access to specialized eye care services, retinal specialists, and advanced treatments for dry AMD may be limited, particularly in rural or underserved areas. Geographic disparities in access to care, socioeconomic barriers, and lack of insurance coverage may prevent some patients from receiving timely diagnosis, treatment, and follow-up care. Improving access to eye care services through telemedicine, community outreach programs, and collaborative care models is essential for addressing disparities in patient outcomes.
Challenges in Patient Recruitment for Clinical Trials: Recruiting and retaining participants for clinical trials in dry AMD can be challenging due to the relatively low prevalence of the disease, stringent eligibility criteria, and competition among clinical trial sponsors. Enrolling a diverse patient population that reflects the heterogeneity of dry AMD and ensuring adequate representation of underrepresented groups (e.g., minorities, older adults) are critical for generalizing trial results and advancing evidence-based practice.
Regulatory and Reimbursement Challenges: Navigating complex regulatory pathways and securing reimbursement for novel therapies in dry AMD pose significant challenges for drug developers and manufacturers. Variability in regulatory requirements across jurisdictions, evolving evidentiary standards, and uncertainty regarding reimbursement coverage and pricing may deter investment in dry AMD drug development. Addressing regulatory and reimbursement challenges requires collaboration among industry stakeholders, regulatory agencies, payers, and patient advocacy groups to streamline approval processes and ensure timely access to innovative therapies.
Future Dry Age-related Macular Degeneration Market Analysis
Analyzing the future of the dry age-related macular degeneration (AMD) market involves considering emerging trends, technological advancements, regulatory developments, and evolving healthcare landscapes. Here's a prospective analysis of the future dry AMD market:
Growing Disease Burden: With the aging population and increasing life expectancy, the prevalence of dry AMD is expected to rise, leading to a growing disease burden and greater demand for effective management strategies. As a result, there will be an increased focus on research, diagnosis, and treatment options to address the needs of individuals with dry AMD.
Advancements in Diagnostic Technologies: Technological innovations in imaging modalities, such as optical coherence tomography (OCT), fundus autofluorescence (FAF), and adaptive optics imaging, will continue to improve the early detection, diagnosis, and monitoring of dry AMD. These advancements will enable more accurate assessment of disease progression, facilitate personalized treatment approaches, and support clinical decision-making.
Precision Medicine Approaches: Advances in genetics, molecular profiling, and precision medicine will enable personalized approaches to dry AMD management. Biomarker discovery efforts and genetic testing may identify individuals at higher risk of disease progression or with specific genetic subtypes of dry AMD, guiding treatment selection and prognosis prediction.
Emerging Therapeutic Modalities: Research into novel therapeutic modalities for dry AMD, including gene therapy, cell-based therapies, and regenerative medicine approaches, will continue to advance. Preclinical and clinical studies exploring the potential of gene editing technologies, stem cell transplantation, and neuroprotective agents aim to address the underlying mechanisms of dry AMD and provide disease-modifying treatments.
Combination Therapies: Combination therapies targeting multiple pathways involved in dry AMD pathogenesis may offer synergistic effects and improved treatment outcomes. Combinations of anti-inflammatory agents, neuroprotective compounds, angiogenesis inhibitors, and immunomodulatory drugs could provide additive or complementary effects, slowing disease progression and preserving vision in patients with dry AMD.
Digital Health Solutions: Digital health solutions, including telemedicine platforms, remote monitoring devices, and mobile applications, will play an increasingly important role in dry AMD management. These technologies enable remote patient monitoring, facilitate home-based vision testing, support patient education and self-management, and enhance communication between patients and healthcare providers.
Regulatory Support for Innovation: Regulatory agencies will continue to provide support and incentives to expedite the development and approval of innovative therapies for dry AMD. Designations such as orphan drug status, fast track designation, breakthrough therapy designation, and priority review designation will accelerate the regulatory review process for promising therapies targeting unmet medical needs in dry AMD.
Healthcare Integration and Access to Care: Integration of eye care services into primary care settings, multidisciplinary care teams, and collaborative care models will improve access to comprehensive care for individuals with dry AMD. Coordinated efforts among ophthalmologists, optometrists, retinal specialists, and primary care providers will optimize patient outcomes and ensure timely diagnosis and treatment.
Patient-Centered Care and Advocacy: Patient advocacy organizations and support groups will continue to play a vital role in raising awareness, promoting education, and advocating for the needs of individuals with dry AMD. Empowering patients, caregivers, and families through education, peer support networks, and access to resources will enhance patient-centered care and improve quality of life.
Economic and Market Dynamics: Economic factors, market competition, and healthcare policies will influence the commercialization and adoption of new treatments for dry AMD. Pricing strategies, reimbursement policies, and market access considerations will impact the availability and affordability of innovative therapies, shaping market dynamics and patient access to care.
Evolving Dry Age-related Macular Degeneration Treatment Outlook
The evolving treatment outlook for dry age-related macular degeneration (AMD) involves a multifaceted approach encompassing advancements in diagnostics, pharmacotherapy, regenerative medicine, and supportive care. Here's an overview of the evolving landscape of dry AMD treatment:
Diagnostics and Early Intervention: Advances in diagnostic imaging technologies, such as optical coherence tomography (OCT), fundus autofluorescence (FAF), and adaptive optics imaging, enable earlier detection and more precise monitoring of dry AMD. Early intervention strategies aim to identify high-risk individuals, detect disease progression, and initiate treatment before irreversible vision loss occurs.
Nutritional Supplements: Dietary supplementation with specific vitamins and minerals, such as vitamins C and E, zinc, copper, lutein, zeaxanthin, and omega-3 fatty acids, has been shown to slow the progression of dry AMD in certain patient populations. Research continues to explore the optimal formulation, dosing regimen, and long-term efficacy of nutritional supplements in preserving vision and reducing the risk of advanced AMD.
Anti-inflammatory Agents: Chronic inflammation plays a key role in the pathogenesis of dry AMD, making anti-inflammatory agents potential therapeutic targets. Drugs targeting inflammatory mediators, such as complement inhibitors, corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), and immunomodulators, aim to suppress retinal inflammation, reduce drusen formation, and prevent disease progression.
Neuroprotective and Anti-oxidant Therapies: Neuroprotective agents and antioxidants may help preserve retinal function and mitigate oxidative stress-induced damage in dry AMD. Compounds such as ciliary neurotrophic factor (CNTF), pigment epithelium-derived factor (PEDF), resveratrol, and coenzyme Q10 have shown neuroprotective effects in preclinical studies and clinical trials, offering potential therapeutic benefits for preserving photoreceptor and retinal pigment epithelial (RPE) cell function.
Angiogenesis Inhibitors: While abnormal blood vessel growth (neovascularization) is characteristic of wet AMD, emerging evidence suggests that angiogenic factors may also contribute to the pathogenesis of dry AMD. Anti-angiogenic agents targeting vascular endothelial growth factor (VEGF), such as aflibercept and ranibizumab, have shown promise in slowing disease progression and reducing geographic atrophy (GA) growth in certain subtypes of dry AMD.
Cell-Based Therapies: Regenerative medicine approaches using cell-based therapies, including stem cell transplantation, retinal pigment epithelial (RPE) cell replacement, and induced pluripotent stem cells (iPSCs), hold promise for repairing damaged retinal tissue and restoring vision in dry AMD. Clinical trials investigating the safety and efficacy of cell-based therapies are underway, with the goal of developing regenerative treatments for advanced dry AMD.
Gene Therapy and Genetic Targeting: Gene therapy strategies aim to correct genetic mutations associated with dry AMD, modulate gene expression, and restore normal cellular function in the retina. Techniques such as gene editing, RNA interference (RNAi), and viral vector delivery systems enable targeted delivery of therapeutic genes to retinal cells, offering potential disease-modifying effects and long-term benefits for individuals with genetic forms of dry AMD.
Drug Delivery Systems: Innovative drug delivery systems, such as sustained-release implants, nanoparticles, microparticles, and hydrogels, enhance the localized delivery of therapeutic agents to the retina, prolonging drug release and reducing treatment frequency. These drug delivery platforms improve treatment efficacy, minimize side effects, and optimize patient compliance in dry AMD management.
Combination Therapies and Multimodal Approaches: Combining multiple therapeutic modalities, such as anti-inflammatory agents, neuroprotective agents, and nutritional supplements, may offer synergistic effects and improved outcomes in dry AMD treatment. Multimodal approaches integrating pharmacotherapy, regenerative medicine, and supportive care aim to address the complex pathophysiology of dry AMD and optimize visual function.
Patient-Centered Care and Supportive Services: Patient-centered care models, low vision rehabilitation programs, and supportive services play a critical role in addressing the psychosocial impact of vision loss and optimizing patient outcomes in dry AMD. Low vision aids, adaptive technologies, vision rehabilitation therapy, and psychosocial support programs help individuals with dry AMD maximize their remaining vision, maintain independence, and improve quality of life.
Role of Companies in the Dry Age-related Macular Degeneration Market
In the Dry Age-related Macular Degeneration market, companies such as Alkeus Pharmaceuticals, Novartis, Molecular Partners, Stealth BioTherapeutics, Regenerative Patch Technologies, Aevitas Therapeutics, NGM Biopharmaceuticals, InflammX Therapeutics, Lineage Cell Therapeutics, Alexion AstraZeneca Rare Disease, Belite Bio, Katairo, Cognition Therapeutics, Apellis Pharmaceuticals, Galimedix Therapeutics, Amarna Therapeutics, 4D Molecular Therapeutics, Aviceda Therapeutics, Isarna Therapeutics, and others play a pivotal role in driving innovation, research, development, and the provision of treatments and therapies for individuals suffering from this chronic inflammatory skin condition. These companies encompass pharmaceutical giants, biotechnology firms, medical device manufacturers, and healthcare service providers, each contributing uniquely to the advancement of Dry Age-related Macular Degeneration management. Pharmaceutical companies lead the charge in developing novel drugs, ranging from topical corticosteroids to biologics targeting specific immune pathways implicated in Dry Age-related Macular Degeneration pathogenesis.
Dry Age-related Macular Degeneration Market Outlook - Key Conclusion and Analysis
The Dry Age-related Macular Degeneration market is undergoing a transformative period, driven by advances in research, innovation in therapeutic approaches, and shifting treatment paradigms. While significant progress has been made in improving outcomes for patients with Dry Age-related Macular Degeneration, several barriers continue to challenge the market's expansion, including high treatment costs, safety concerns, and regulatory hurdles. Looking ahead, personalized medicine, novel therapeutic targets, and digital health solutions are poised to shape the future of Dry Age-related Macular Degeneration management, offering new hope for patients and caregivers alike. Efforts to address these challenges and capitalize on emerging opportunities will be critical in advancing the field and ultimately improving the lives of individuals living with Dry Age-related Macular Degeneration.
Get a more detailed overview, at: Dry Age-related Macular Degeneration Market Outlook and Forecast
#Dry Age-related Macular Degeneration market#Dry Age-related Macular Degeneration#Dry Age-related Macular Degeneration market share#Dry Age-related Macular Degeneration treatment market#Dry Age-related Macular Degeneration market size
0 notes