#GMP Testing Service Market Market GMP Testing Service Market Market Size GMP Testing Service Market Market Share GMP Testing Service Market
Text
GMP Testing Service Market: Global Industry Analysis and Forecast 2023 – 2030
Global GMP Testing Service Market was valued at USD 1.17 Billion in 2021 and is expected to reach USD 1.88 Billion by the year 2028, at a CAGR of 6.97% .
Major factors contributing to the market expansion for GMP testing services include the pharmaceutical industry's explosive growth and the ongoing trend of drug and device development. The trend of biopharmaceutical companies developing more medications and devices is being driven by the increasing burden of chronic diseases, rising healthcare costs, and the impending patent expiration of blockbuster drugs. This is projected to propel the expansion of the studied industry. The launch of novel medications with cutting-edge features and strategic partnerships, as well as the growing R&D efforts of pharmaceutical companies, are anticipated to fuel the market's expansion.
Get Full PDF Sample Copy of Report: (Including Full TOC, List of Tables & Figures, Chart) @
https://introspectivemarketresearch.com/request/15848
Updated Version 2024 is available our Sample Report May Includes the:
Scope For 2024
Brief Introduction to the research report.
Table of Contents (Scope covered as a part of the study)
Top players in the market
Research framework (structure of the report)
Research methodology adopted by Worldwide Market Reports
Moreover, the report includes significant chapters such as Patent Analysis, Regulatory Framework, Technology Roadmap, BCG Matrix, Heat Map Analysis, Price Trend Analysis, and Investment Analysis which help to understand the market direction and movement in the current and upcoming years.
Leading players involved in the GMP Testing Service Market include:
Eurofins Scientific, PPD Inc., Microchem Laboratory, Sartorius AG, North American Science Associates Inc., Laboratory Corporation of America Holdings (Covance Inc.), Sotera Health (Nelson Laboratories LLC), Almac Group, Pace Analytical, Wuxi AppTec, Intertek Group PLC, Charles River Laboratories and Others major players.
If You Have Any Query GMP Testing Service Market Report, Visit:
https://introspectivemarketresearch.com/inquiry/15848
Segmentation of GMP Testing Service Market:
By Type
Product Validation Testing
Bioanalytical Services
Packaging
Shelf-Life Testing
Others
By End-User
Pharmaceutical & Biopharmaceutical Companies
Medical Devices Company
By Regions: -
North America (US, Canada, Mexico)
Eastern Europe (Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe)
Western Europe (Germany, UK, France, Netherlands, Italy, Russia, Spain, Rest of Western Europe)
Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New Zealand, Rest of APAC)
Middle East & Africa (Turkey, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa)
South America (Brazil, Argentina, Rest of SA)
What to Expect in Our Report?
(1) A complete section of the GMP Testing Service market report is dedicated for market dynamics, which include influence factors, market drivers, challenges, opportunities, and trends.
(2) Another broad section of the research study is reserved for regional analysis of the GMP Testing Service market where important regions and countries are assessed for their growth potential, consumption, market share, and other vital factors indicating their market growth.
(3) Players can use the competitive analysis provided in the report to build new strategies or fine-tune their existing ones to rise above market challenges and increase their share of the GMP Testing Service market.
(4) The report also discusses competitive situation and trends and sheds light on company expansions and merger and acquisition taking place in the GMP Testing Service market. Moreover, it brings to light the market concentration rate and market shares of top three and five players.
(5) Readers are provided with findings and conclusion of the research study provided in the GMP Testing Service Market report.
Our study encompasses major growth determinants and drivers, along with extensive segmentation areas. Through in-depth analysis of supply and sales channels, including upstream and downstream fundamentals, we present a complete market ecosystem.
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
Acquire This Reports: -
https://introspectivemarketresearch.com/checkout/?user=1&_sid=15848
About us:
Introspective Market Research (introspectivemarketresearch.com) is a visionary research consulting firm dedicated to assisting our clients to grow and have a successful impact on the market. Our team at IMR is ready to assist our clients to flourish their business by offering strategies to gain success and monopoly in their respective fields. We are a global market research company, that specializes in using big data and advanced analytics to show the bigger picture of the market trends. We help our clients to think differently and build better tomorrow for all of us. We are a technology-driven research company, we analyse extremely large sets of data to discover deeper insights and provide conclusive consulting. We not only provide intelligence solutions, but we help our clients in how they can achieve their goals.
Contact us:
Introspective Market Research
3001 S King Drive,
Chicago, Illinois
60616 USA
Ph no: +1-773-382-1047
Email: [email protected]
#GMP Testing Service#GMP Testing Service Market#GMP Testing Service Market Size#GMP Testing Service Market Share#GMP Testing Service Market Growth#GMP Testing Service Market Trend#GMP Testing Service Market segment#GMP Testing Service Market Opportunity#GMP Testing Service Market Analysis 2023
0 notes
Text
GMP Testing Service Market Growth
Global GMP Testing Service Market was valued at USD 1.17 Billion in 2021 and is expected to reach USD 1.88 Billion by the year 2028, at a CAGR of 6.97% .

The GMP testing services are relevant to the various manufacturers in food and beverages industry, cosmetics production, pharmaceutical drugs and medical devices industry. GMP testing services are a must when it comes to obtaining a license for initiating the production where mostly safety and quality is evaluated. For export-oriented businesses adhering to the GMPs of place of export are an obligation.
The report is based on a thorough examination of the whole worldwide GMP testing service market, as well as all of its sub-segments, using exhaustive classifications. With contributions from industry professionals across the value chain, in-depth research and assessment are developed from premium primary and secondary information sources.
#GMP Testing Service Market#GMP Testing Service Market Size#GMP Testing Service Market Share#GMP Testing Service Market Analysis
0 notes
Text
In-Depth Look at Itsuwa: A Decade of Excellence in Vaping Innovation

Company Overview
Since its inception in 2007, Itsuwa has carved out a prestigious niche as a leading electronic cigarette manufacturer. Under the visionary leadership of CEO Jasmin Guo, the company has become an indispensable OEM/ODM partner for renowned global brands, pushing the boundaries of vaping technology and service.
Excellence in Manufacturing
Itsuwa prides itself on adhering to the strictest GMP and ISO9001 standards. The facility is equipped with state-of-the-art testing labs and maintains an impressively low defect rate of under 0.3%, ensuring top-tier product safety and quality.
Product Innovation
From the well-loved VapeSoul to the VOOM and AWAK series, Itsuwa is not just a manufacturer but an innovator at heart. Over 25 dedicated engineers drive its R&D, tailoring products to enhance every aspect of the vaping experience, catering to both casual users and enthusiasts seeking robust mod systems.
Operational Excellence
Itsuwa is synonymous with operational efficiency, guaranteeing rapid product delivery within just seven days through trusted couriers. The flexibility in order size handling demonstrates their commitment to customer satisfaction, big or small.
Research and Development
The R&D team, powered by over 30 passionate engineers, continuously innovates to elevate vaping experiences while optimizing costs. Their goal is not just to keep pace but to set the pace in the ever-evolving vaping landscape.
Customer-Centric Approach
Itsuwa’s dedication to customer relations is evident in their quick response time and regular client interactions. By valuing each customer's journey, Itsuwa builds lasting partnerships based on trust and mutual growth.
Embracing the Future
With the vaping industry in constant flux, Itsuwa remains at the forefront, committed to innovation and quality. As they advance, they continue to be the preferred choice for businesses navigating the complex vape market.
For a deeper dive into Itsuwa’s innovative product lines and exemplary services, make sure to visit their website. Stay tuned to our blog for the latest updates and expert insights in the vaping world. Join us on this journey and let’s vape smartly and stylishly together!
Read the full article
0 notes
Text
0 notes
Text
Potent Compound Development Market Size, Volume, Demand, Outlook | BIS Research
Potent Compound Development, it's imperative to underscore the significance of our collective efforts in advancing pharmaceutical research and ultimately improving patient care. Potent compounds represent a cornerstone of modern medicine, offering the potential to target specific molecular pathways with precision and efficacy.
The global HPAPI market is projected to reach $84.20 billion by 2033 from $27.44 billion in 2023, growing at a CAGR of 11.86% during the forecast period 2023-2033.
Download the report and get a better understanding Click Here!
Potent Compound Development Overview
Potent compound development refers to the systematic process of discovering, optimizing, and synthesizing chemical compounds with high biological activity at low concentrations.
These compounds, known as potent compounds or high-potency drugs, demonstrate strong efficacy in modulating specific molecular targets involved in disease pathways.
The development of potent compounds involves a multidisciplinary approach, including molecular biology, medicinal chemistry, pharmacology, and preclinical and clinical research.
Key Stages in Potent Compound Development are as follows
Target Identification and Validation
Lead Compound Identification
Preclinical Testing
Clinical Trials
Regulatory Approval
Market Launch
Download our sample page click here !
Market Segmentation
Segmentation 1: based on Type
(i) by type of synthesis
(ii) by type of manufacturing
Segmentation 2: based on Therapeutic Area
(i) Oncology
(ii) Neurology
(iii) Infectious Diseases
(iv) Autoimmune Diseases
(v) Cardiovascular Diseases
Segmentation 3: based on Population Segmentation
(i) Pediatric Population
(ii)Geriatric Population
(iii) Rare Diseases
Segmentation 4: based on Geographic Segmentation
(i) Emerging Markets
(ii) Developed Markets
Segmentation 5: based on Technology
(i) Biologicals
(ii) Small Molecules
Segmentation 6: by Regulatory Environmental Segmentation
(i) FDA Approved Drugs
(ii) EMA Approved Drugs
.
Key Companies are as follows
Almac Group
Asymchem Inc.
BASF Pharma Solutions
CARBOGEN AMCIS
CordenPharma International
Market Trends and Drivers
Increasing Incidence of chronic and complex diseases
Advancements in biotechnology and drug discovery technologies
Rise of Immunotherapies and targeted therapies
Drug development and innovation
Recent Developments in the Highly potent API Market
In December 2022, Almac concluded the initial phase of its good manufacturing practice (GMP) active pharmaceutical ingredient (API) facility expansion as part of a multi-million-pound investment program.
In October 2022, Asymchem Inc., a prominent global provider of contract development and manufacturing services, and AUM Biosciences (AUM), a global biotech company in the clinical stage, with a focus on the discovery, acquisition, and development of next-generation targeted oncology therapeutics, jointly declared the successful conclusion of their inaugural GMP production campaign for AUM601.
Click here to have a look at Life Sciences & Biopharma page !
Key Question Answers
Q What is the estimated global market size for the highly potent API market?
Q What are the different types of highly potent API market available in the market?
Q How has the COVID-19 outbreak affected the future trajectory of the highly potent API market?
Q What are the key trends influencing the global highly potent API market, and what is their potential for impacting the market?
Q What does the patent landscape of the global highly potent API market look like? Which year and country witnessed the maximum patent filing between January 2020 and December 2023?
Conclusion
Potent compound development stands at the forefront of pharmaceutical innovation, driving the discovery and commercialization of highly effective therapies for a wide range of diseases and medical conditions.
Potent compound development will continue to be driven by collaboration, innovation, and a commitment to addressing unmet medical needs.
0 notes
Text
Importance of Stability Test Chamber in Industries
What is Stability Test Chamber?
In industries where precise environmental conditions are paramount, Stability Test Chambers play a crucial role. These chambers provide controlled settings for testing and storing products, ensuring their stability and integrity over time. However, with a myriad of stability chamber manufacturers in the market, choosing the right one can be a daunting task. In this blog post, we delve into the realm of stability chamber manufacturers, exploring key considerations and tips to help you find the perfect fit for your specific requirements.

Importance of Stability Test Chambers:
Before delving into manufacturers, it’s essential to grasp the significance of stability chambers in various industries:
1. Pharmaceutical: Stability chambers are vital for pharmaceutical companies to assess the stability of drugs and ensure their efficacy and safety over their shelf life.
2. Food and Beverage: In the food and beverage industry, stability chambers help maintain product quality by simulating storage conditions and evaluating shelf-life parameters.
3. Research and Development: Stability chambers are indispensable in research laboratories for conducting experiments and studies that require precise temperature and humidity control.
Key Considerations When Choosing a Manufacturer:
When evaluating stability chamber manufacturers, consider the following factors to make an informed decision:
1. Reputation and Experience: Opt for manufacturers with a proven track record and extensive experience in designing and producing stability chambers. Look for testimonials, reviews, and case studies to gauge their reputation and reliability.
2. Customization Options: Assess the manufacturer’s ability to tailor stability chambers to meet your specific needs. Look for flexibility in terms of chamber size, temperature range, humidity control, and additional features.
3. Quality and Compliance: Ensure that the manufacturer adheres to industry standards and regulations, such as FDA, ICH, and GMP guidelines. Look for certifications and quality assurance processes to guarantee the reliability and accuracy of their chambers.
4. Technical Support and Service: Choose a manufacturer that offers comprehensive technical support, training, and after-sales service. Consider factors such as warranty coverage, maintenance plans, and availability of spare parts.
Top Stability Test Chamber Manufacturer:
While numerous manufacturers specialize in stability chambers, several industry leaders stand out for their quality, reliability, and innovation:
1. Ferrotek Equipment: Ferrotek Equipment is recognized for its high-performance stability chambers featuring innovative design, precise control, and user-friendly interface, catering to the stringent requirements of pharmaceutical and research laboratories.
2. ESPEC North America: With a legacy of over 60 years, ESPEC is renowned for its precision-engineered stability chambers, offering a wide range of models tailored to diverse industries and applications.
3. Weiss Technik: As a global leader in environmental testing solutions, Weiss Technik delivers cutting-edge stability chambers known for their advanced technology, accuracy, and durability.
Conclusion:
Choosing the right stability chamber manufacturer is paramount to ensuring the integrity and reliability of your testing and storage processes. By considering factors such as reputation, customization options, quality, and technical support, you can make an informed decision that aligns with your specific needs and objectives. Whether you prioritize precision, reliability, or versatility, partnering with a trusted manufacturer is the first step towards achieving optimal results and compliance in your industry.
#stability chamber manufacturer#stability chamber manufacturer in India#stability test chamber#stability test chamber manufacturer#stability test chamber manufacturer in India#Top supplier of stability test chamber#stability chamber supplier#ferrotek equipment
1 note
·
View note
Text
Quality Without Compromise: GMP Certification for Food Safety

GMP Certification in Lebanon - Good manufacturing practice, or GMP for short, is a method that guarantees that goods constantly meet quality requirements. The Food and Drug Administration (FDA) established GMP requirements to reduce the hazards of manufacturing products like medications and supplements.
A product's consistent production and control by quality standards is guaranteed by a GMP (good manufacturing practice) certification. By testing the finished product, it reduces hazards of pharmaceutical production that cannot be completely removed. In addition to guaranteeing the safety, effectiveness, and quality of food and pharmaceutical products, GMP certification is essential for boosting global credibility and public trust.
What are the Benefits of GMP Certification?
Improved Product Quality: GMP Registration in Cambodia guarantees that goods are produced by strict quality control guidelines, resulting in goods that are consistently dependable and of a high caliber. By doing this, the chance of product recalls and noncompliance is reduced.
Enhanced Consumer Confidence: Businesses can increase consumer trust in their products by complying with established GMP requirements. This is especially crucial in sectors where safety and effectiveness are essential, such as the food and pharmaceutical industries.
Regulatory Compliance: GMP Certification assists businesses in fulfilling the legal and regulatory obligations set by global health authorities. Compliance lowers the chance of legal trouble and expensive fines and penalties.
Market Expansion: Getting GMP Certified is frequently a need to go international. It creates export prospects because many nations demand that international firms adhere to GMP guidelines to sell goods within their borders.
Operational Improvement: procedure enhancements that boost operational efficiency are usually the result of the GMP Certification procedure. Optimized procedures decrease inefficiencies and good output, and can eventually result in substantial financial benefits.
How much does the GMP Certification Cost?
The kind of service, size of business, operational complexity, and certification body selection are some of the variables that might affect the GMP cost in Oman. The chosen certification organization and the range of its services have an impact on the total cost of certification for Good Manufacturing Practices, in addition to industry standards that may affect Cost.
What are the steps in the GMP Certification Audit process?
Preparation and Documentation Review: To start, the business needs to compile and arrange all the documentation that proves it complies with GMP regulations. SOPs (standard operating procedures), employee training records, and quality management systems are examples of this.
Initial examination: To comprehend the facility's procedures, spot any significant deficiencies, and organize the main audit, an auditor from the certifying organization of GMP Audit in Brazil does an initial examination. This phase assists businesses in identifying the areas in which they must make improvements to comply with GMP requirements.
On-Site Audit: During the on-site audit, auditors check that GMP regulations are followed, examine the production processes, and check the physical facilities. They watch activities, speak with employees, and determine whether procedures comply with legal standards.
Reporting: The auditor creates a thorough report outlining any non-compliances or observations that require attention following the on-site audit. The organization can obtain certification by following the well-defined approach outlined in this study.
Corrective Actions and Follow-Up: The business must take corrective action if non-compliances are discovered. When these changes are implemented, an additional audit might be planned to confirm that all problems have been fixed and the business is now in compliance with GMP requirements, which would result in certification.
How and Where to Obtain the GMP Certification Services?
It is recommended to work with a respectable consulting company with a strong worldwide reputation, like B2Bcert, while seeking GMP certification services in Algeria. B2Bcert, an internationally renowned organization that specializes in audits, consultancy, and validation services, is equipped to help you navigate the GMP certification procedure and its related regulations. Contact the professionals at [email protected] for help or questions regarding GMP certification.
#GMP Certification in Lebanon#GMP Certification in Cambodia#GMP Certification in Oman#GMP Certification in Brazil#GMP Certification in Algeria
1 note
·
View note
Text
“GMP Certification for Sustainable Success"

GMP Certification in Netherlands is a unique mark of excellence in the manufacturing industry. Start a journey of quality assurance that will change your life. GMP Certification is a dedication to excellence rather than just a set of guidelines. Embrace detailed quality controls that go above and beyond expectations to elevate your operations. Delivering products of unwavering safety and consistency is encapsulated in this internationally recognized certification. Accept a culture that combines creativity and precision to put your company on the path of legal compliance and trust. Securing GMP Certification guarantees that your products stand out in a standard-driven environment; it's more than just a stamp.
GMP Certification’s Unique Market Benefits
Inspections of quality: GMP Services in Yemen guarantees that production procedures follow strict guidelines for quality, producing reliable, secure, and superior products.
Customer Confidence: People are becoming more aware of the safety and quality of the products they buy. Customers' confidence and honesty are bolstered by GMP Certification, which may improve sales and foster brand loyalty.
Market Access: Because GMP Certification attests to a company's dedication to quality and safety, it is frequently a requirement for entering certain domestic and foreign markets.
Operational Efficiency: Production costs are lowered and profitability is increased when effective and standardized manufacturing methods are adopted, which is encouraged by GMP Certification.
Advantage over competitors: Companies with GMP certification enjoy a leg up in the industry. Their leadership position in quality and compliance is enhanced by their accreditation, which sets them apart from rivals.
Universal Recognition: Trade and collaboration across nations are facilitated by GMP's international recognition. Businesses operating in international markets may find the GMP Certification to be a useful resource.
GMP Certification Budget Breakdown: Planning for Success
GMP Cost in Maldives prices might change depending on the industry and size of the organization. Typical costs include internal audit resources, staff training, implementation consultant fees, documentation systems, infrastructure upgrades, equipment amends, and certification body fees. The prices of certification include application, evaluation, and yearly renewal fees. To the entire investment, there are additional costs associated with third-party testing, compliance maintenance, and continuous improvement initiatives. It is advised that companies get detailed cost estimates from consultants and certifying bodies according to their particular certification requirements and operational context.
GMP Certification and the Audit Journey
Utilization:A certification body receives an application for GMP Audit in Delhi,along with the required documentation of its activities and procedures.
Examining the Document:To make sure the organization's quality management system paperwork complies with GMP requirements, the certification body examines it.
Conclusions of the audit:A report outlining the auditors' findings is provided; this report may include information on areas of compliance, non-compliance, and areas that could use improvement.
Corrective Measures:In most cases, the organization must provide corrective action plans to remedy concerns found if non-compliance is found.
Choice Regarding Certification:The certification body determines whether the organization satisfies GMP requirements by assessing the audit results and corrective actions.
Issue of Certification:The certifying body grants the GMP certifying if the organization satisfies the GMP standards.
How to Secure GMP Certification Consultants in the Netherlands
For further information on How to Find GMP Certification Consultants in New York please visit www.b2bcert.com, the official website of our company. Alternatively, if you need assistance with GMP training or consulting solutions in the Netherlands, send an email to [email protected] with your requirements. At B2Bcert, value added comes first in order to comprehend requirements and choose the most accurate and cost-effective procedure for your business to obtain CE certification.
0 notes
Text
Agilus Diagnostics IPO Date, Price, GMP, Company profile, financials & risks: Dec 23
New Post has been published on https://wealthview.co.in/agilus-diagnostics-ipo/
Agilus Diagnostics IPO Date, Price, GMP, Company profile, financials & risks: Dec 23

Agilus Diagnostics IPO: Agilus Diagnostics Limited, formerly known as SRL Limited, is a leading diagnostics service provider in India. It boasts the largest network of laboratories in the country, offering a comprehensive range of tests across various specialties. Agilus operates in a rapidly growing Indian diagnostics market, fueled by rising healthcare awareness, increasing disposable income, and an aging population.
Agilus Diagnostics IPO Key Details:
Offer type: Offer for Sale (OFS) by existing shareholders (International Finance Corporation, NYLIM Jacob Ballas India Fund III LLC, and Resurgence PE Investments Ltd) – no fresh capital being raised by the company.
Issue Dates: Yet to be announced.
Offer Size: Up to 142.3 million equity shares.
Price Band: Not yet determined.
Recent News Updates:
Agilus filed its Draft Red Herring Prospectus (DRHP) with SEBI in September 2023, seeking approval for the IPO.
The company reported a consolidated revenue of Rs 1,347 crore for FY23, down from Rs 1,604 crore in FY22. However, profitability remained relatively stable at Rs 116 crore, suggesting potential resilience.
The Indian diagnostics market is projected to reach USD 27 billion by 2026, providing significant growth opportunities for Agilus.
Agilus Diagnostics IPO: Offer Details
Securities Offered:
This is an Offer for Sale (OFS) by existing shareholders. Therefore, no new securities (equity shares, bonds, etc.) will be issued by Agilus Diagnostics Limited. Existing shareholders, namely International Finance Corporation, NYLIM Jacob Ballas India Fund III LLC, and Resurgence PE Investments Ltd, will be offloading a portion of their holdings through the IPO.
Reservation Percentages:
The specific reservation percentages for different investor categories are yet to be finalized by the Securities and Exchange Board of India (SEBI). However, the typical distribution in Indian IPOs follows this pattern:
Retail Individual Investors (RIIs): 35%
Qualified Institutional Buyers (QIBs): 50%
Non-Institutional Investors (NIIs): 15%
The final percentages might vary depending on regulatory requirements and investor demand.
Minimum Lot Size and Investment Amount:
The minimum lot size for the IPO, which determines the number of shares you can subscribe to in a single application, will be announced closer to the issue date. It is typically set at a level affordable for retail investors, ranging from 100 to 200 shares.
The minimum investment amount will depend on the minimum lot size and the final issue price, which are yet to be confirmed. For example, if the minimum lot size is 100 shares and the issue price is Rs 100 per share, then the minimum investment amount would be Rs 10,000 (100 shares * Rs 100).
Agilus Diagnostics Limited Company profile:
History and Operations:
Founded in 1995, Agilus boasts a rich legacy of over 28 years in the Indian diagnostics landscape.
Initially known as SRL Limited, it rebranded to Agilus Diagnostics in 2023.
Operates a vast network of over 413 laboratories spread across 25 states and 5 union territories in India, as of March 2023.
Offers a comprehensive range of diagnostic tests catering to various medical specialties, including pathology, biochemistry, microbiology, immunology, and genetics.
Provides services directly through its own labs, partnerships with hospitals and clinics, and home sample collection facilities.
Market Position and Market Share:
Ranked second in terms of revenue from operations among diagnostic companies in India for Fiscal 2023 (Source: CRISIL Report).
Holds the title of India’s largest chain of diagnostic laboratories by geographic presence (Source: Agilus Diagnostics website).
As of March 2023, boasts the largest network of NABL accredited laboratories in India with 43 accredited labs (Source: CRISIL Report).
Prominent Brands, Subsidiaries, and Partnerships:
The “SRL” brand still enjoys strong recognition in many regions, even post-rebranding.
In April 2023, acquired Lifeline Laboratory, a reputed player in Delhi NCR, further strengthening its reach.
Partners with various hospitals and clinics across India to provide diagnostic services within their premises.
Key Milestones and Achievements:
Pioneered the concept of standardized and affordable diagnostic services in India.
Achieved significant growth through organic expansion and strategic acquisitions.
Awarded prestigious certifications like NABL for its labs, demonstrating commitment to quality standards.
Recognized for its contribution to healthcare by various industry bodies and publications.
Competitive Advantages and Unique Selling Proposition (USP):
Vast network: Reaches a broader patient base, providing accessibility and convenience.
NABL accreditation: Ensures high-quality and reliable diagnostic tests.
Comprehensive service range: Caters to diverse medical needs under one roof.
Technology focus: Implements cutting-edge technology for accurate and efficient testing.
Strong brand recognition: Builds trust and customer loyalty.
Future Growth Prospects and Earnings Drivers:
Market growth: The Indian diagnostics market is projected to reach USD 27 billion by 2026, offering substantial growth potential for Agilus.
Expansion plans: The company aims to expand its network further, targeting geographically under-penetrated areas and strategic acquisitions.
Focus on preventive care: Increasing awareness of preventive healthcare is expected to drive demand for diagnostic services, benefiting Agilus’ comprehensive offerings.
Technology adoption: Continued investment in automation and advanced testing technologies will improve efficiency and attract tech-savvy customers.
Potential Risks and Concerns for Agilus Diagnostics IPO:
Investing in any IPO, including Agilus Diagnostics Limited, involves inherent risks that investors should carefully consider before making a decision. Here are some key areas of concern:
Industry Headwinds:
The Indian diagnostics industry faces potential challenges like regulatory changes, pricing pressures from government healthcare initiatives, and competition from new players. These factors could affect Agilus’ growth and profitability.
Company-Specific Challenges:
While Agilus boasts a strong network and financial position, there are some aspects to consider:
The recent revenue decline raises questions about future growth momentum.
Reliance on existing tests might limit diversification and exposure to newer, high-growth segments.
Integration of acquired entities like Lifeline Laboratory needs careful management to maintain operational efficiency.
Financial Health:
Although debt-free, Agilus’ financial performance in FY23 showed a revenue decline. The final P/E ratio after the issue price is determined will be crucial to assess valuation and potential risks.
Investors should thoroughly analyze the company’s financial statements and future projections provided in the DRHP before making a decision.
Red Flags:
Any unexpected negative news about the company or the industry during the pre-IPO or listing period could raise red flags and impact investor sentiment.
A significantly high GMP compared to industry benchmarks might indicate unrealistic expectations and potential for price correction after listing.
Agilus Diagnostics Limited Draft Offer Documents filed with SEBI
Also Read: How to Apply for an IPO?
#Allotment Details#Company Profile#Financial Performance#Investment#IPO date#Lot Size#UPCOMING IPO#IPO#News
0 notes
Text
GMP Testing Service Market: Global Industry Analysis and Forecast 2023 – 2030
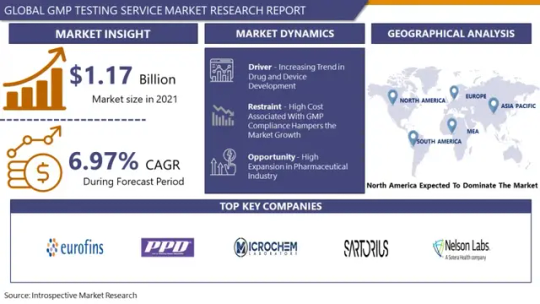
Global GMP Testing Service Market was valued at USD 1.17 Billion in 2021 and is expected to reach USD 1.88 Billion by the year 2028, at a CAGR of 6.97% .
Major factors contributing to the market expansion for GMP testing services include the pharmaceutical industry's explosive growth and the ongoing trend of drug and device development. The trend of biopharmaceutical companies developing more medications and devices is being driven by the increasing burden of chronic diseases, rising healthcare costs, and the impending patent expiration of blockbuster drugs. This is projected to propel the expansion of the studied industry. The launch of novel medications with cutting-edge features and strategic partnerships, as well as the growing R&D efforts of pharmaceutical companies, are anticipated to fuel the market's expansion.
Get Full PDF Sample Copy of Report: (Including Full TOC, List of Tables & Figures, Chart) @
https://introspectivemarketresearch.com/request/15848
Updated Version 2024 is available our Sample Report May Includes the:
Scope For 2024
Brief Introduction to the research report.
Table of Contents (Scope covered as a part of the study)
Top players in the market
Research framework (structure of the report)
Research methodology adopted by Worldwide Market Reports
Moreover, the report includes significant chapters such as Patent Analysis, Regulatory Framework, Technology Roadmap, BCG Matrix, Heat Map Analysis, Price Trend Analysis, and Investment Analysis which help to understand the market direction and movement in the current and upcoming years.
Leading players involved in the GMP Testing Service Market include:
Eurofins Scientific, PPD Inc., Microchem Laboratory, Sartorius AG, North American Science Associates Inc., Laboratory Corporation of America Holdings (Covance Inc.), Sotera Health (Nelson Laboratories LLC), Almac Group, Pace Analytical, Wuxi AppTec, Intertek Group PLC, Charles River Laboratories and Others major players.
If You Have Any Query GMP Testing Service Market Report, Visit:
https://introspectivemarketresearch.com/inquiry/15848
Segmentation of GMP Testing Service Market:
By Type
Product Validation Testing
Bioanalytical Services
Packaging
Shelf-Life Testing
Others
By End-User
Pharmaceutical & Biopharmaceutical Companies
Medical Devices Company
By Regions: -
North America (US, Canada, Mexico)
Eastern Europe (Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe)
Western Europe (Germany, UK, France, Netherlands, Italy, Russia, Spain, Rest of Western Europe)
Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New Zealand, Rest of APAC)
Middle East & Africa (Turkey, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa)
South America (Brazil, Argentina, Rest of SA)
What to Expect in Our Report?
(1) A complete section of the GMP Testing Service market report is dedicated for market dynamics, which include influence factors, market drivers, challenges, opportunities, and trends.
(2) Another broad section of the research study is reserved for regional analysis of the GMP Testing Service market where important regions and countries are assessed for their growth potential, consumption, market share, and other vital factors indicating their market growth.
(3) Players can use the competitive analysis provided in the report to build new strategies or fine-tune their existing ones to rise above market challenges and increase their share of the GMP Testing Service market.
(4) The report also discusses competitive situation and trends and sheds light on company expansions and merger and acquisition taking place in the GMP Testing Service market. Moreover, it brings to light the market concentration rate and market shares of top three and five players.
(5) Readers are provided with findings and conclusion of the research study provided in the GMP Testing Service Market report.
Our study encompasses major growth determinants and drivers, along with extensive segmentation areas. Through in-depth analysis of supply and sales channels, including upstream and downstream fundamentals, we present a complete market ecosystem.
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
Acquire This Reports: -
https://introspectivemarketresearch.com/checkout/?user=1&_sid=15848
About us:
Introspective Market Research (introspectivemarketresearch.com) is a visionary research consulting firm dedicated to assisting our clients to grow and have a successful impact on the market. Our team at IMR is ready to assist our clients to flourish their business by offering strategies to gain success and monopoly in their respective fields. We are a global market research company, that specializes in using big data and advanced analytics to show the bigger picture of the market trends. We help our clients to think differently and build better tomorrow for all of us. We are a technology-driven research company, we analyse extremely large sets of data to discover deeper insights and provide conclusive consulting. We not only provide intelligence solutions, but we help our clients in how they can achieve their goals.
Contact us:
Introspective Market Research
3001 S King Drive,
Chicago, Illinois
60616 USA
Ph no: +1-773-382-1047
Email: [email protected]
#GMP Testing Service#GMP Testing Service Market#GMP Testing Service Market Size#GMP Testing Service Market Share#GMP Testing Service Market Growth#GMP Testing Service Market Trend#GMP Testing Service Market segment#GMP Testing Service Market Opportunity#GMP Testing Service Market Analysis 2023
0 notes
Text
The Dynamic Evolution of Infertility Medicines Companies in India
Introduction:
In the realm of healthcare, the global infertility drug market is witnessing a substantial surge, projected to reach new heights by the end of 2024. With a market size valued at US$3.8 billion in 2017 and an anticipated Compound Annual Growth Rate (CAGR) of 5.8%, the demand for infertility drugs is on the rise. This surge is attributed to the increasing prevalence of infertility, making it a critical concern for today’s generation. For those in the pharmaceutical industry, particularly experts, wholesalers, stockists, and retailers, the infertility range presents not only a challenge but also a lucrative career prospect.
This blog aims to guide pharma professionals in their quest for the right company to collaborate with. Choosing a reputable and trustworthy pharma company is paramount for success in the competitive industry. Here, we present a comprehensive list of the top infertility pharma companies in India, each distinguished by its commitment to quality and services.
List of Top Infertility Pharma Companies in India:
Adorefem:
Adorefem stands out as a professional name in the infertility and gynaecology range, earning a stellar reputation in the Indian pharma market. The company’s focus on delivering high-quality fertility/IVF range, including tablets, capsules, injections, syrups, and soft gels, sets it apart. Adorefem’s commitment to providing DCGI approved quality medicines, utilizing cutting-edge technology, and being supported by a skilled team of professionals showcases its supremacy in the industry.
https://www.adorefem.com/wp-content/uploads/2020/09/logo-1.png
Surewin Healthcare:
Surewin Healthcare stands tall as a seasoned player in the industry, offering a wide array of Gynecology and Infertility Medicines throughout India. The company has garnered acclaim for delivering certified, high-quality products and providing opportunities for aspiring entrepreneurs through Pharma Franchise options. With a commitment to transformative growth, trustworthy relationships, and pharmaceutical excellence, Surewin Healthcare has become a beacon of reliability in the market.
Krishlar Pharmaceuticals:
Based in Chandigarh, Krishlar Pharmaceuticals takes pride in providing top-notch gynecological products at affordable prices. Renowned for its commitment to quality and effectiveness, the company has established itself as a trusted name in the pharmaceutical domain. Krishlar Pharmaceuticals collaborates with esteemed partners, ensuring the delivery of unparalleled services and high-quality gynecological items.
Solace Biotech Limited:
As a well-known brand in the Indian market, Solace Biotech Limited has maintained its reputation for a decade by consistently delivering high-quality Gynae products. The company holds WHO, GMP, and ISO 9001:2008 certifications, assuring the distribution of DCGI-approved pharmaceuticals across the nation. Solace Biotech Limited remains dedicated to adhering to stringent quality standards and conducting regular inspections to ensure the safety and efficacy of its products.

Gladfem:
Gladfem takes a dual approach to gynecological medicine, offering both allopathic and ayurvedic options. Operating in GMP and WHO-certified facilities, Gladfem ensures the production of defect-free, pure, effective, and safe medications. Specializing exclusively in the Gynaecology medicine range, Gladfem boasts a specialized staff, laboratory, testing facilities, and production plant, underscoring its commitment to excellence.
Moruf Life Sciences:
Positioned as India’s Best Gynae PCD Franchise Company, Moruf Life Sciences adopts a quality-driven strategy to ensure customer satisfaction. The company embraces technological advancements and invests in extensive research and development to provide cutting-edge Gynae medication. Moruf Life Sciences’ dedication to quality is evident in its top-notch products and services.
Femcorp:
As one of India’s leading gynecology companies, Femcorp has emerged as an ISO-certified pharma franchise company. The company’s focus on high-quality work, advanced formulation facilities, and ethical business practices make it a standout player in the industry. Femcorp also offers enticing services such as free promotional gadgets, high overall income, and no large deal aims, creating a favorable environment for franchisees.
Texas Therapeutics:
Texas Therapeutics has rapidly gained recognition as the top firm in India for Gynecological Infertility Medicines for PCD Franchise. Compliant with ISO, GMP, DCGI, WHO, and FSSAI quality requirements, the company’s infertility medications are well-received in the market. Texas Therapeutics provides lucrative franchise opportunities for those seeking to enter the pharmaceutical market.
India bulls Pharmaceuticals:
Established in 2016 in New Delhi, India bulls Pharmaceuticals has become a prominent player in the pharmaceutical market. With 35+ GMP guaranteed manufacturing facilities, the company formulates and supplies a diverse range of pharmaceutical items, including highly sought-after women’s healthcare products.
Albia Biocare:
Albia Biocare, a well-known company in the Gynecology Pharma Franchise sector, prioritizes customer satisfaction by exceeding expectations in quality, packaging, and delivery. The company values market inputs and feedback, striving to meet ever-changing demands with unwavering dedication.
Rx Biotech:
With the motto of “Living Healthy With Quality Life,” Rx Biotech is a dynamic Gynecology PCD pharma franchise firm based in New Delhi. The company aims for excellence in service quality, improved goods, creative marketing, and on-time delivery, making it a reliable partner for those seeking fertility solutions.
The journey towards finding the right partner in the realm of infertility medicines in India requires a careful assessment of each company’s offerings. These top infertility pharma companies have demonstrated their commitment to quality, efficacy, and service excellence. As pharmaceutical professionals embark on this crucial decision-making process, the hope is that this comprehensive list guides them toward a fruitful collaboration, ensuring success in meeting the rising demand for high-quality infertility range among customers.
Unveiling the Transformative Landscape of Infertility in India:
The narrative of infertility in India is undergoing a transformative shift, propelled by societal and lifestyle changes impacting fertility rates. As this silent struggle gains visibility, the demand for effective and advanced infertility medicines has surged. This blog unravels the layers of this evolving landscape, shedding light on the pioneering companies that specialize in providing solutions for couples embarking on the path to parenthood.
The Rise of Infertility Medicines in India: A Paradigm Shift
The historical context of infertility treatments in India reveals a paradigm shift in societal perspectives and medical advancements. From a once hushed conversation, infertility has moved into the spotlight, and companies are rising to the occasion. This section explores the changing dynamics, emphasizing the increasing openness to seeking medical assistance and the crucial role played by innovative medicines.
Pioneering the Way: India’s Leading Infertility Medicines Company
In the heart of this transformative landscape stands India’s leading infertility medicines company, a beacon of hope for countless couples. This segment introduces the trailblazers, their commitment to research, and their mission to redefine the possibilities of conception. It delves into the company’s ethos, highlighting the fusion of traditional values with a commitment to cutting-edge medical solutions.
Harmony of Tradition and Technology: The Unique Approach of Indian Infertility Medicines
What sets Indian infertility medicines apart is the harmonious integration of traditional wisdom with modern technology. Ayurveda, with its centuries-old principles, converges with state-of-the-art pharmaceutical innovations, creating a synergy that addresses not just the physical but also the emotional aspects of infertility. This section explores the unique approach, emphasizing the holistic nature of the solutions offered.
Innovations Shaping the Future: Breakthroughs in Infertility Medicines
The future of infertility medicines in India is marked by continuous innovation. From advanced hormonal therapies to groundbreaking reproductive technologies, this segment unravels the latest breakthroughs that are reshaping the landscape. It delves into how these innovations are not just addressing infertility but also enhancing the chances of successful conception.
Patient-Centric Care: Navigating the Emotional Landscape of Infertility
Beyond the pharmaceutical advancements, what defines India’s leading infertility medicines company is its commitment to patient-centric care. This section explores the empathetic approach, recognizing the emotional toll of infertility. From personalized treatment plans to support systems, the company places the emotional well-being of couples at the forefront.
Accessible Hope: The Reach and Impact of Indian Infertility Medicines
Accessibility is a key aspect of India’s success in the realm of infertility medicines. This section explores how these medicines are reaching diverse socio-economic segments, breaking barriers and making hope accessible to a broader demographic. It delves into initiatives that ensure affordability without compromising on the quality of care.
Cultural Sensitivity in Infertility Solutions: Adapting to Diverse Needs
Understanding the cultural nuances surrounding infertility is paramount. Indian infertility medicines companies are adept at adapting solutions to diverse cultural contexts. This segment explores the importance of cultural sensitivity, recognizing that the journey towards parenthood is deeply intertwined with cultural beliefs and practices.
Educating and Empowering: The Role of Indian Infertility Medicines Companies
In the quest for parenthood, knowledge is empowerment. Indian infertility medicines companies go beyond providing treatments; they play a pivotal role in educating couples. This section explores the educational initiatives, emphasizing the importance of informed decisions and the empowerment that comes with understanding one’s fertility journey.
Conclusion:
As we draw the curtains on this exploration of infertility medicines in India, the narrative that emerges is one of hope, innovation, and unwavering commitment. India’s leading infertility medicines company stands as a testament to the transformative power of blending ancient wisdom with modern science. The holistic approach, innovative breakthroughs, and patient-centric care converge to redefine the possibilities of conception.
In the vast landscape of infertility, this pioneering company not only addresses physical challenges but also acknowledges and supports the emotional journey. It goes beyond providing medicines, offering accessible hope, adapting to diverse cultural needs, and playing a crucial role in educating and empowering couples.
The story is not just about overcoming challenges but about ushering in a new era where the dream of parenthood becomes a tangible reality. Through a harmonious blend of tradition and technology, India’s leading infertility medicines company continues to shape a future where every couple can embark on the journey of parenthood with optimism and assurance.
Read More: Top Pharma Companies for Infertility Range
Source: https://www.adorefem.com/the-dynamic-evolution-of-infertility-medicines-companies-in-india/
0 notes
Text
0 notes
Text
0 notes
Text
List Of PCD Pharma Franchise Companies In India
List Of PCD Pharma Franchise Companies In India – Stop Googling “List Of PCD Pharma Franchise Companies In India” now! Because you are already on the right platform. As we all agree, the pharma industry is one of the faster-growing sectors and also offers great business opportunities to its members.

Highly increasing health diseases, rising pollution, and infectious microorganisms increase the demand for pharma products. These days, harmful viruses, impure and unhealthy foods raise the risk of health issues and which is why people demand high-quality medical drugs that give them fast and quick results. When it comes to pcd pharma business it is very important to choose the right and trusted company and also it is a little bit difficult task. Feel free now because here we give you the complete information related to well-named and leading companies that are known for their high-quality products and services.
What are the Perks of Pharma Franchise Business?
If you are interested to run a small as well as middle-sized business then you must know the benefits of a pharma franchise business because it will assist you increase your knowledge or capital in the long run. The following are the perks of pharma franchise business are given below:
Low-risk factors and low investment are required.
Monopoly rights advantage.
Higher opportunity for growth and development.
Business liberty
Low marketing cost
No high competition
Free to expand your business.
Greater earnings and profits.
List Of Top PCD Pharma Franchise Companies In India
The Indian pharmaceutical industry is rising day by day and undoubtedly there are so many companies available that provide the pcd pharma franchise opportunities all across the nation but choosing the best for yourself is a challenging task. So we decided we will help you by providing the well-known companies’ name that is highly suitable for you and your business. Now, let’s move to the List of Top Pharma PCD Companies in India that are given below:
1. Eraas International
Eraas International is a well-renowned and trustworthy pharmaceutical company. This company comes in the Top PCD Pharma Franchise Company In India. They provide a wide range of pharma products that are available in different forms like tablets, capsules, syrups, ointments, injections, sachets, and so on. Also, they have an experienced and knowledgeable team that follows all the rules and guidelines of WHO and GMP and after complete testing, they deliver all their products to its customers.
Furthermore, Eraas International uses modern technology and high-tech machinery for producing pharma products and medicines. If you are seeking the best company for your new business then join hands with Eraas International because we are also counted as one of the Top PCD Pharma Franchise Companies in India.
High-quality Products
Promotional Tools
Low Investment
Timely Delivery
Attractive Packaging
Monopoly Rights
Growth Opportunities
Low-Risk Involvement
Huge Profit
Huge Marketing Network
Different Ranges of Pharma Drugs
Contact Details
Company Name – Eraas International
Contact Number – +91 98181 29750, 011-45644901
Email Address – [email protected]
Registered Address – I-103, Lower Ground Floor, Kirti Nagar, New Delhi, India, Pin – 110 015
2. Elkos Healthcare Pvt. Ltd.
Elkos Healthcare Pvt. Ltd. is the leading and one of the Best PCD Pharma Companies in India. The main aim of this company is to provide affordable products that are approved by DCGI. The packaging material that they use is very top-quality and they are also capable of delivering all the products on time.
Address: Plot NO.246 HSIIDC Industrial EstateAlipur, Barwala, Panchkula-134118Haryana, India.
3. Albia Biocare
Albia Biocare is trusted and counted as one of the Top 10 PCD Pharma Companies in India having years of experience in this field. Their pharma range is quite impressive in terms of dosage forms and product segments both. Albia Biocare has skilled professionals and experienced quality controllers.
Address: 3/3, Subhash Nagar, Manimajra, Chandigarh, Haryana 160101
4. Novalab Healthcare
Novalab Healthcare comes in the List of Top 10 PCD Pharma Franchise Companies in India. They also provide promotional tools free of cost to the franchise associates including MR Bags, Diaries, Pen Sets, Notepads, Reminder Cards, and so on. Novalab Healthcare delivers its products to all locations in India on time.
Address – SCO 831, 2ND Floor, NAC Manimajra, Chandigarh 160101.
Conclusion
Here to conclude, we give you the complete List Of PCD Pharma Franchise Companies In India. All the given companies are ISO-certified companies and working in this industry for several years. We hope the list listed above will help you to choose the best option for your needs.
Frequently Asked Questions (FAQs)
Question 1. Which company is the Best PCD Pharma Franchise Company in India?
Answer. Eraas International is the Best PCD Pharma Franchise Company in India.
Question 2. What are the reasons why you should start a pharma franchise business?
Answer. Here are a few reasons that are given below:
Monopoly Rights
Profitable Business
Low Risk with Investment Requirements
Higher Opportunities
Provide Large Established Platform
#Best PCD Pharma Franchise Company in India#List of Top 10 PCD Pharma Franchise Companies in India#Top PCD Pharma Franchise Company In India#Best PCD Pharma Companies in India
0 notes
Text
0 notes