Text
Researchers Find Herpes Infections In Human Brains Marked Via Alzheimer's Condition
The viruses, best known for inducing a distinctive skin rash in young children, are abundant in brain tissue from people with Alzheimer's disease, a team of scientists reports Thursday in Neuron. The group also found evidence that the viruses may interact with brain cells in a sense that may accelerate the illness.
"Our theory is that they put gas to the fire," states Joel Dudley, an author of the study and an associate professor of genetics and genomic sciences in the Icahn School of Medicine at Mt. Sinai in new york.
The finding adds credence into a decades-old thought that a disease can result in Alzheimer's disease. In addition, it indicates it might be possible to block or slow Alzheimer's using antiviral medications, or drugs that regulate how immune cells within the brain react to an infection.
"The data are extremely intriguing, but fall short of demonstrating a direct causal character," he says. "And when viral infections have been playing a part, they are not the only real performer."
youtube
Even so, the research provides strong evidence that viral diseases can help determine the course of Alzheimer's, Hodes says.
Just like lots of scientific discoveries, this one has been an collision.
He and a team of researchers were using genetic information to search for differences between healthy brain tissue and brain tissue from people who died with Alzheimer's.
The aim was to identify new targets for medications. Instead, the team kept discovering signs that brain tissue in Alzheimer's patients contained higher levels of viruses.
"When we started assessing the differences, it just kind of came screaming out at us from the statistics," Dudley says.
The team discovered that levels of 2 human herpes viruses, HHV-6 and HHV-7, were around twice as high in brain tissue from people with Alzheimer's disease. They confirmed the finding by assessing data from a consortium of mind banks.
These herpes infections are very common, and can cause a skin rash called roseola in young children. But the viruses also can get into the brain, where they could remain dormant for decades.
Once the researchers knew the viruses were correlated with Alzheimer's they started trying to figure out how a virus could influence the path of a cardiovascular disorder. That supposed distinguishing connections between the virus genes and other genes in the brain cells.
"We mapped on the social network, if you may, of which genes that the viruses are friends with and that they're speaking to within the brain," Dudley says. In nature, he saysthey wanted to know:"If the viruses are tweeting, who is tweeting back?"
And what they discovered was that the herpes virus enzymes have been interacting with enzymes known to increase a person's risk for Alzheimer's.
They also found these Alzheimer's risk genes seem to generate a individual's brain more vulnerable to infection with both herpes viruses.
However, only having herpes virus within the brain isn't enough to cause Alzheimer's, Dudley says. Something should activate the viruses, and that induces them to start replicating.
It's not clear what triggers the stimulation, Dudley states, though he suspects some kind of shift in the inner functions of cells.
Once the germs are becoming active, they seem to affect things such as the accumulation of these plaques and tangles in the brain linked to Alzheimer's disease.
The herpes viruses additionally appear to trigger an immune response in certain brain cells, Hodes states. These cells are a part of an early immune system which has been implicated in Alzheimer's.
Most previous efforts to prevent or treat Alzheimer's have entailed trying to reduce the plaques and tangles associated with the disease. Those efforts have failed to enhance brain function even when they accomplished their immediate goal.
Those"distressing and disappointing failures" suggest it is time to get some fresh strategies, Hodes states. Along with the new analysis indicates at least two.

One would be to provide antiviral drugs to people with elevated levels of herpes virus in their brains. The Institute on Aging is currently funding a study to examine this approach in people in the first stages of Alzheimer's, Hodes says.
Another strategy is to avoid the brain immune cells from reacting to the virus in several ways that accelerate Alzheimer's, Hodes states. That is catchy, he says, cause simply disabling the mind of immune cells could be detrimental.
Nevertheless, Hodes remains optimistic.
"The more we know about the disease process and the further aims we can address," he says,"the higher the probability we are going to slow or stop the progression of Alzheimer's disease."
0 notes
Text
Expected Herpes Virus Vaccines Might Be Available In a short time
Medical investigators could be finally closing in on an effective vaccine to the genital sores brought on by the herpes simplex virus, even following exciting results from recent research in guinea pigs and monkeys.

Human security trials of one vaccine candidate, composed of three essential viral proteins, which could begin in less than 18 months.
youtube
One of the most frequent sexually transmitted diseases, genital herpes is distinguished by itchy, itchy, itchy, and painful lesions on the skin on and around the genitals and inside the anus, urethra, and anus that resolve and recur intermittently. The disease, caused chiefly by the HSV-2 herpes simplex breed and somewhat less often by HSV-1, is incurable, and past attempts to come up with both vaccines and treatments have neglected.
Herpes simplex's trickiness stems from its ability to prevent the body's immune system -- also circulating anti-viral drug representatives -- by rapping in neurons of the peripheral nervous system whose endings, or axons, are at the genital skin. As many as two out of 3 individuals permanently harbor HSV-1, and one in five have HSV-2; and though only a fraction of people experiences observable symptoms, even people without active lesions might pass it on to the others. Each of HSV infections are known to put people at greater risk of contracting HIV if exposed to this virus.
The latest offender, created by a team in Louisiana State Universitywhich uses a strain of live-attenuated virus which has been engineered with disabling mutations in the protein that enables the virus to enter axons. Their assessment in guinea pigs, published this week in the journal Vaccine, revealed that none of the nine guinea hens who received injections (plus two boosters) of the engineered strain became infected after exposure to a highly virulent HSV-2 breed, whereas most nine of the unvaccinated animals failed.
According to New Scientist, direct writer Konstantin Kousoulas reports further, as-of-yet unpublished experiments indicate that the vaccine may also purify the immune system in guinea pigs already infected with a sterile herpes breed and that the vaccine is safe in anti inflammatory primates.
"We think it has enormous potential as a therapeutic approach," Kousoulas told the magazine.
Cases of herpes simplex 2 illnesses along with also a visualization of the virus itself. Fitzpatrick's Color Atlas and Synopsis of Clinical Dermatology, Eighth Edition, 2017
In January 2017, the University of Pennsylvania team behind the protein fragment vaccine candidate published breakthrough findings from a set of tests in animals.
Their study revealed that introducing immune system into three viral surface glycoproteins -- both involved in immune evasion and one that assists in entering neurons -- can curb the growth of genital lesions and block the virus from successfully replicating in the vaginal tissues of guinea pigs, a sign that would not be transmissible to other individuals.
After exposure to a infamously pathogenic HSV-2 breed from sub-Saharan Africa, 97 percent of hens that received only one shot and 99% of those that have an booster were symptom-free. The group's evaluations in macaques theorized a routine of three vaccine shots can be perfect for inducing a strong immune response against potent viral particles in primates for example.
In October 2017, lead researcher Dr Harvey Friedman said that his staff will be working with drug organizations to initiate human testing. He declared that although animal models have a spotty history in terms of predicting vaccine success in people, he's got a positive outlook on the capacity of his or her candidate.
0 notes
Text
Long-Awaited Herpes Virus Vaccines Could Be Ready Shortly
Medical researchers may be finally closing in about an effective vaccine for the genital sores caused by the herpes simplex virus, even after stimulating results from recent studies in guinea pigs and monkeys.
Human safety trials of a single vaccine candidate, consisting of three essential viral proteins, which could begin in under 18 months.
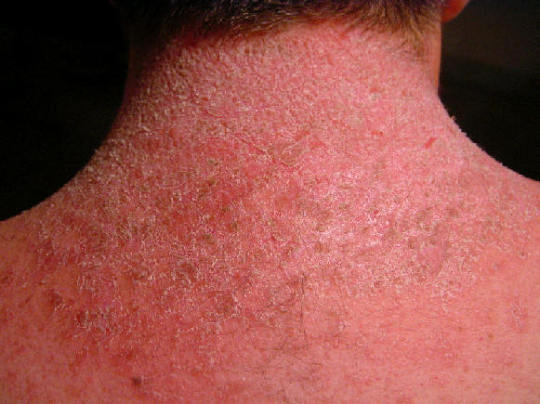
Among the most common sexually transmitted diseases, genital herpes is characterized by itchy, itchy, inflamed, and painful lesions on the skin on and around the genitals and also within the vagina, urethra, and anus that solve and recur intermittently. The infection, caused chiefly from the HSV-2 herpes simplex strain and somewhat less often by HSV-1, is incurable, and past attempts to come up with both treatments and vaccines have failed.
youtube
Herpes simplex's trickiness stems from its ability to evade the body's immune system -- and circulating anti-viral drug representatives -- by rapping in nerves of the peripheral nervous system whose endings, or axons, are in the genital skin. As many as two out of three people permanently harbor HSV-1, and one in five have HSV-2; and though only a fraction of people experiences visible symptoms, even those with active lesions might pass it on to others. All HSV diseases are known to place people at higher risk of contracting HIV if exposed to this virus.
The latest offender, created by a group in Louisiana State University, utilizes a strain of live-attenuated virus which was designed with disabling mutations in the protein which enables the virus to enter axons. Their assessment in guinea pigs, released this week in the journal Vaccine, showed that none of the nine guinea pigs who received shots (and two boosters) of the engineered breed became infected following exposure to a highly virulent HSV-2 strain, whereas most nine of those unvaccinated animals did.
According to New Scientist, direct author Konstantin Kousoulas reports that further, as-of-yet unpublished experiments indicate that the vaccine can also purify the immune system in guinea hens infected with a sterile herpes breed and the vaccine is secure in anti inflammatory primates.
"We believe it has tremendous potential as both a therapeutic approach," Kousoulas advised the magazine.
Cases of herpes simplex 2 illnesses along with a visualization of the virus .
In January 2017, the University of Pennsylvania team behind the protein fragment vaccine offender published breakthrough findings by a set of evaluations in animals.
Their study demonstrated that introducing that immune system to three viral surface glycoproteins -- both involved in immune evasion and one that aids in penetrating neurons -- can suppress the growth of genital lesions and block the virus from successfully replicating in the vaginal tissues of guinea pigs, a sign that wouldn't be transmissible to other people.
After exposure to a infamously pathogenic HSV-2 strain from sub-Saharan Africa, 97% of hens that received one shot and 99% of those that have an booster were symptom-free. The team's evaluations in macaques theorized a regimen of three vaccine shots can be perfect for inducing a strong immune response against potent viral particles from primates for example.
In October 2017, lead researcher Dr Harvey Friedman stated that his staff will be working with drug organizations to initiate human testing. He conceded that although animal models have a spotty history in terms of predicting vaccine success in people, he's got a favorable outlook on the potential of his candidate.
0 notes