Text
IIT JEE preparation tips : COMPLETE MATHEMATICS
IIT JEE preparation tipsn: COMPLETE MATHEMATICS FOR JEE MAIN 2020
Summary:”Entire Mathematics for JEE Main 2020” judiciously combines practice and theory, with the ideal emphasis on solved exercises and examples. The whole syllabus is covered in 28 chapters that adhere strictly to the present pattern of this examination. Salient features: – Lots of solved examples confirmed by training questions – Chapter-wise past years’ questions entirely solved – hints, tricks and methods supplied through notes and opinions – solution of former questions are inserted to every . There are total 1328 pages.
how to prepare for JEE mains
About the Author
Ravi Prakash, Associate Professor (Retd.), Rajdhani College, University of Delhi Ajay Kumar, Professor of Mathematics, University of Delhi Usha Gupta, Associate Professor (Retd.), Rajdhani College, University of Delhi.

IIT JEE preparation tips : Comprehensive Mathematics for JEE Advanced 2020
Produced according to the most recent syllabus of JEE Advanced, comprehensive Mathematics for JEE Advanced guides the students through all the significant theories of math in detail. This publication is an extensive source for the next phase of their JEE examinations.
Click here to
28 core chapters together with pertinent theory and practice issues two. Solved examples and exercise issues have been classified into the following classes: (a) Single Correct Answer Form Questions (b) Multiple Correct Answer Form Questions (c) Matrix Match Form Questions (Id )Assertion–Reason Form Questions (e)Comprehension-Type Questions (Id ) Integer-Answer Form Questions (gram ) Exercises at the end of the chapter are split into 2 levels of difficulty — Level 1 and Level 2 (h) Lots of fresh solved examples in Addition to new exercises (I) Topic shrewd IIT-Advanced questions because 1978 are included and their answers (j) 2018 JEE Advanced queries Together with the Answer Key and complete solutions supplied at the end of the publication (k) Please log on www.mhhe.com/jeeadvancedcomprehensivemathematics to get (l) Two entirely solved model evaluation papers (two ) Solutions to each of the level 2 queries in the publication
0 notes
Link
JEE mains tips and tricks : JEE 2020 Application Form for January semester was extended until 10th October. Candidates may fill program via internet mode. Stay tuned with examSumo for quickest upgrades.
0 notes
Link
The National Testing Agency has significantly altered the exam blueprint for the JEE Main 2020. Registrations for the exam will be available from 3-30 September 2019.
0 notes
Link

Learn essential tips and tricks that can help you crack the JEE mains and advanced. Our experts provide study plan, important topics, mock test and tips to prepare for JEE mains. Click here to get best IIT JEE preparation tips!!
0 notes
Link

Learn essential tips and tricks that can help you crack the JEE mains and advanced. Our experts provide study plan, important topics, mock test and tips to prepare for JEE mains. Click here to get best IIT JEE preparation tips!!
0 notes
Link

Learn essential tips and tricks that can help you crack the JEE mains and advanced. Our experts provide study plan, important topics, mock test and tips to prepare for JEE mains. Click here to get best IIT JEE preparation tips!!
0 notes
Text
JEE mains tips and tricks : Top 6 Ways To Stay Motivated
JEE mains tips and tricks : Preparation for IIT JEE requires plenty of effort and determination. You need to be prepared to go through several sleepless nights to complete the huge JEE syllabus. But just finishing the syllabus isn’t sufficient! If you would like an edge over your counterparts you want to step your game up. This implies practising and revising faithfully while adhering to a meticulous research program which admits your own strengths and takes care of your flaws. You need to constantly inspire yourself to keep pushing till that test date.Let us take a peek at a few of the very best ways which you may keep yourself inspired as you prepare yourself for IIT JEE.

JEE mains tips and tricks : Top Strategies to stay inspired for IIT JEE
Favorable self-affirmation
At any time you get stuck trying to comprehend or resolve a issue, you are inclined to feel demotivated. At that stage, you want to remind yourself that the pupils that are preparing for JEE are going through precisely the exact same battle. You have to tell yourself you can not stop. Rather, identify the true problem that’s stopping you from going ahead and invent the perfect approach to make the most of your time and energy.
Regardless of the autumn, you have to pick yourself up, dust yourself off and keep walking (or analyzing, in this instance ). Ignoring your errors will not help . Come to terms with them and also be decided ot never create them .
JEE mains tips and tricks : Exciting Electrical Engineering
Make self-driven
You yourself will be the best man to do this. As soon as you identify your driveway, it becomes easier to take care of the struggles, mistakes, etc.. You have to understand you could rely on to cope with whatever curve balls that the test yells at you. In reality, understanding that’ll make you more inspired.
Be in good Friend Circle
Both negative and positive energy can be contagious so eliminate any unwanted energy. If you’re amidst good business, it is only natural you will be in great spirits. Ensure to surround yourself with strong-willed, self-driven individuals and you’ll discover that simply being in their center is inspiring enough.
JEE mains tips and tricks : Differential Equation
Picture a Larger Picture
Getting ready for an extreme examination like JEE, it’s without a doubt you will get bored and upset whilst searching through its immense syllabus and sophistication of these subjects. At the point your creativity enters the picture. Ponder at the time once you buy a degree from a IITs, the heavy bundle and the sort of luxury life you will lead. These imaginations will enhance your confidence and inspire you to research further.
Reward yourself every day
Maintain a daily target; it might be that you complete a specific topic daily or perhaps you resolve a set of challenging issues that day. It might be a ice-cream or thirty minutes of the Language sitcom you adore so much. This may rejuvenate your brain and sub-consciously encourage you to attain daily objectives.
Use these 5 tips to enter the top engineering schools of India by draining the aggressive JEE main and advanced. With the assistance of examSumo. You can research all vital theories with extreme clarity and clinic all of the relevant questions to contact the concepts. You might even check yourself with the very unique mock evaluation series that offer detailed analysis which will be able to enable you to get a clear image of your own performance. These mock evaluations help pupils understand their weakness and strengths together with assisting them to devise an effective approach to ace the exam.
0 notes
Text
JEE mains tips and tricks : JEE 2020 Application Form
JEE mains tips and tricks : JEE 2020 Application Form for January semester was extended until 10th October. Candidates may fill program via internet mode. Stay tuned with examSumo for quickest upgrades. Picture correction in program form was started.

For More Information : how to prepare for JEE advanced
NTA introduced new modifications in JEE Mains examination pattern. Candidates can assess the newest changes below presented in JEE Mains 2020 evaluation:
In the year 2020, the examination will be run individually to get BE/B.Tech Candidates may look for all of the classes or individually.
Application Fee continues to be increased compared to the past year. It may be compensated via online style .
Changes in Assessment routine: New modifications are introduced in the test pattern of JEE Main 2020. The amount of queries in the examination paper was diminished into 25 queries (per topic ) compared to last year (30 queries ). Additionally, besides the MCQs from the newspaper there’ll be queries whose answers must be full as numerical worth .
From this season, the paper routine of B.Plan was individually introduced at JEE Main.
NTA JEE Main 2020 will likely be conducted double each year from the months of January & April in online CBT style .
The age limitation to use for JEE Mains was eliminated. Now applicants satisfying the eligibility criterion are able to submit an application for the examination no matter the age.
For JEE Main Paper 2 (B.Arch/B.Plan), It’s compulsory for the applicants to possess Mathematics, Physics and Chemistry subjects.
Aadhar card isn’t compulsory to use for JEE Mains 2020 entry examinations.
Students appearing among the best 2,45,000 qualified students will be qualified to look for JEE Advanced.
NTA has established over 3400 Practice centres from the nation for its preparation of their pupils. Diabetic pupils can take water bottlefruits and sugar pills within the test centre.
JEE mains tips and tricks : Photoelectric Effect
JEE Mains 2020 Exam Dates
Below We’ve Supplied All of the official JEE Main 2020 dates for a Variety of events Happening at the January session and April session:
January Session: Events Dates 2020 Notification release August 2019 Release of Application Form 3rd September 2019 Image correction started 13th September 2019 Last date to submit the application form 10th October 2019 (Extended) Last date of fee submission & image uploading 11th October 2019 Application correction window 14th – 20th October 2019 Availability of Admit card 6th December 2019 JEE Main 2020 Exam 6th – 11th January 2020 Declaration of Results 31st January 2020 April Session: Events Dates 2020 Release of Online Application 7th February 2020 Image correction window opens In the month of February 2020 Availability of mock test In the month of February 2020 Last date to submit application 7th March 2020 Last date of fee submission & image uploading 2
nd
week of March 2020 Downloading of Admit card 16th March 2020 JEE Mains 2020 Exam 3rd – 9th April 2020 Announcement of Result 30th April 2020
Candidates may assess several important details below about JEE Main 2020 Program form:
The Program form filling procedure for the January session was launched from 3rd September 2019 by NTA. April Session enrollment will begin from 7th February 2020.
The comprehensive online registration procedure comprise a number of measures — Online Registration, Filling the Program online, Uploading of Images, Payment of Program fee and Printing the Program.
Last date to submit the application form for the January session is 10th October 2019. For the April session, applicants may submit the form until 7th March 2020.
Nominees are permitted to submit just 1 application form. Several forms from 1 candidate will get refused.
JEE mains tips and tricks : Mechanics
Application Fee:
Mode of Payment: Applicants may pay the fee from on line style through internet banking/debit card/credit card, UPI & PAYTM.
No offline mode can be obtained to cover the program fee.JEE Main 2020 Application fee for a Variety of classes is given in the table below:
Papers Category Application Fee Exam Center in India Exam Center Outside India B.E./B.Tech
or
B.Arch
or
B.Planning Gen/Gen-EWS/ OBC-NCL candidates Boys – Rs.650
Girls – Rs.325 Boys – Rs.3000
Girls – Rs.1500 SC/ ST/ PwD/ Transgender candidates Boys – Rs.325
Girls – Rs.325 Boys – Rs.1500
Girls – Rs.1500 B.E./B.Tech & B. Arch
or
B.E./B.Tech & B. Planning
or
B.E./B.Tech, B. Arch &
B.Planning
or
B.Arch & B.Planning Gen/Gen-EWS/ OBC-NCL candidates Boys – Rs.1300
Girls – Rs.650 Boys – Rs.6000
Girls – Rs.3000 SC/ ST/ PwD/ Transgender candidates Boys – Rs.650
Girls – Rs.650 Boys – Rs.3000
Girls – Rs.3000
JEE Main 2020 Application Correction
For all candidates enrolled successfully, the board will offer JEE Main Program correction centre to fix mistakes. It is going to be one-time centre for those pupils to make corrections from the facts of program form. Correction in uploaded pictures was launched .The program correction window will open out of 14th into 20th October 2019 for the January session enrollment. Pupils will have the ability to correct information in the program through online manner only. To make correction from the program, students need to pay another quantity of commission .Correction from the program has to be accomplished by the candidates prior to the final date of form entry.
JEE mains tips and tricks : Solid State
JEE Main 2020 Eligibility Criteria
Candidates Should Undergo the JEE Main Eligibility Grade , before Completing the application form:
General Eligibility:
Qualifying Assessment: Implementing candidate should have handed 10+2 degree examination or its equivalent in 2018 or 2019 from a recognized board or college.
Appearing Candidates: Candidates that are looking in course 12th examination may also apply.
Diploma Holders: Nominees that are holding diploma may also use for admissions just in IITs.
Qualifying Topics: For B.E/B.tech, candidate within their own 12th degree needs to have passed at least of five topics using Physics and Mathematics as mandatory subjects together with Biology, Chemistry, Biotechnology or some other specialized Profession.
For B.Plan: For B.Planning, students should have passed their 12th degree with Mathematics subject.
Age Criterion: there’s no age limitation prescribed to use for JEE Main 2020. Total Efforts: there’ll not be any attempt limitation.
Institutions wise Qualification:
To get Entrance into IITs. NITs, CFTIs along with other associations through CSAB, pupils have procured 75 percent (For ST/SC — 65 percent ) marks or have to be under high 20 percentile in 12th degree examinations.
On the grounds of All India Rank of this candidate procured in the exam, entrance will be supplied.
0 notes
Link

Learn essential tips and tricks that can help you crack the JEE mains and advanced. Our experts provide study plan, important topics, mock test and tips to prepare for JEE mains. Click here to get best IIT JEE preparation tips!!
0 notes
Link

Learn essential tips and tricks that can help you crack the JEE mains and advanced. Our experts provide study plan, important topics, mock test and tips to prepare for JEE mains. Click here to get best IIT JEE preparation tips!!
0 notes
Link

Learn essential tips and tricks that can help you crack the JEE mains and advanced. Our experts provide study plan, important topics, mock test and tips to prepare for JEE mains. Click here to get best IIT JEE preparation tips!!
0 notes
Link

Learn essential tips and tricks that can help you crack the JEE mains and advanced. Our experts provide study plan, important topics, mock test and tips to prepare for JEE mains. Click here to get best IIT JEE preparation tips!!
0 notes
Link

Learn essential tips and tricks that can help you crack the JEE mains and advanced. Our experts provide study plan, important topics, mock test and tips to prepare for JEE mains. Click here to get best IIT JEE preparation tips!!
0 notes
Text
Important topics for JEE mains : The photoelectric effect
The photoelectric effect
In the first third of the twentieth century, some peculiarities of the electron began to be known in phenomena that settled the nascent theories of relativity and quantum mechanics. Among them, we can highlight the manifestations known as the photoelectric effect and the Compton effect, two forms of interaction between electrons and electromagnetic radiation.
Click here to how to prepare for JEE mains
In 1887, the German physicist Heinrich Hertz (1857-1894) accidentally discovered that ultraviolet light modified the voltage at which sparks were produced between metal electrodes. The German Philipp Lenard (1862-1947) described this phenomenon, called the photoelectric effect, as the emission of electrons by metallic surfaces when visible or ultraviolet light hits them, and he arrived at two basic conclusions:
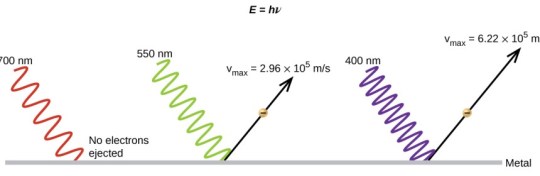
1.The maximum kinetic energy that the emitted electrons can reach does not depend on the intensity of the incident radiation.
2.In the photoelectric effect, the emission of electrons is instantaneous.
Important topics for JEE Mains :Trigonometry
The Bohr-Sommerfeld Atomic Model
The atomic model devised by Ernest Rutherford at the beginning of the 20th century described the hydrogen atom as a system composed of a massive nucleus of positive electric charge and minimum dimensions around which a negative electron moved. When this model proved insufficient, Niels Bohr introduced a series of quantum postulates that established a new conceptual framework for the development of atomic theory.
The hydrogen atom
If the hydrogen atom is considered as a set of nucleus and electron subjected to the laws of the dynamics of the central forces, the total energy and the angular momentum of the electron should be governed by the following expressions:
where r is the radius of the orbit of the electron, m its mass and Z the atomic number of hydrogen (expressed in symbolic form, although its value is 1).
Important topics for JEE Mains :Algebra
These expressions explain the mechanical behavior of the system, but not its electromagnetic properties. According to classical electromagnetism, if an electron emits radiation, it will inevitably fall under the influence of the atomic nucleus.
Model of Bohr-Sommerfeld
To understand the behavior of the hydrogen atom, the Danish Niels Bohr (1885-1962) incorporated to the previous model considerations of the quantum theory. Bohr supposed that the electron can only describe certain circular orbits around the nucleus, which he called stationary and which he identified with integers.
When an electron emits radiation, it moves from a stationary orbit n to another n¿, and the difference between its energies corresponds to the energy of the photon-emitter:
Since the number of possible orbits of the electron is discrete, so is the set of electromagnetic frequencies that it can emit. If an electron absorbs a photon, it acquires energy and moves to an orbit further away from the nucleus, and if it emits it, it loses energy and falls into an orbit closer to the nucleus.
Important topics for JEE Mains : Qualitative Analysis
He also proposed that the allowed orbits would be those whose angular momentum L was a multiple of the constant “h”, which means: L = h n , being n = 1, 2, 3 … In this way, the radii of the Bohr stationary orbits and the associated energy levels would be:
The German physicist Arnold Sommerfeld (1868-1951) completed this atomic model of Bohr considering that the described orbits were not circular, but elliptical, and developed the corresponding corrections.
Matter Waves by Louis De Broglie
Albert Einstein’s work on the photoelectric effect showed that electromagnetic waves are made up of elementary particles called photons. In reverse, the Frenchman Louis De Broglie predicted in 1924 that the material corpuscles of the exterior of the atoms, the electrons, should also show a wave behavior. The experimental verification of the particle and wave duality of electrons, which arrived a few years later, closed the circle of one of the most seductive proposals of quantum physics: everything that exists is, at the same time, wave and matter.
The controversies over the nature of light that had focused scientific debates for more than two centuries were resolved in 1905 when Albert Einsten, in his interpretation of the photoelectric effect, came to reconcile the two hypotheses handled and, until then, considered incompatible:
1.The wave, according to which the light radiation is simply a disturbance that moves in space.
2.The corpuscular, which held that light is made up of material corpuscles capable of interacting with matter.
Einstein concluded that light and, by extension electromagnetic waves, are both corpuscle and wave, since they are composed of massless and uncharged particles, called photons, which propagate in space as a wave motion, exchanging energy with the environment.
Important topics for JEE Mains : Mechanics
In a speculative study, which did not respond to any observed reality that had to be explained, the Frenchman Louis de Broglie (1892-1987) played with the possibility that, like photons, electrons also have the same wave duality and corpuscle.
De Broglie waves
In a work published in 1924, De Broglie started with a comparison between the properties of the photon and the electron to assume that the latter particle could have energy-frequency and wave-momentum linear relationships analogous to the first, and expressed as:
being a vector that shares direction with the wave vector .
Starting from the relativistic hypothesis, an equivalence could be established between energy and the linear moment of the electron considered as a wave and as a material particle, from which it would be deduced that:
De Broglie wavelength
From the comparison of the magnitudes of the behavior of the electron understood as a wave and as a particle, we obtain a value for the wavelength that the wave motion associated with the electron that is given by:
where v is the velocity of the particle and m is its mass. This magnitude, called De Broglie’s wavelength, increases with decreasing speed, and vice versa.
Important topics for JEE Mains :2D, 3D & Vectors
If applied to the postulate of the Bohr atomic model (see t60), which holds that the orbits of electrons in atoms can only have certain radii quantified, it follows that:
According to this formula, the allowed (stationary) orbits in the Bohr model would be those whose radius was equal to an integer number of de Broglie wavelengths.
As for detecting wave behavior in light, it was necessary to handle dimensions of the order of their wavelength (for example, gratings that would cause diffraction patterns as light interferences), to observe the effects of waves associated with matter particles of very small mass and moving at low speed, for example, the electrons themselves, must be used. In these particles it would be possible to obtain de Broglie wavelength values of the order of a few tenths of a nanometer.
0 notes
Link

Learn essential tips and tricks that can help you crack the JEE mains and advanced. Our experts provide study plan, important topics, mock test and tips to prepare for JEE mains. Click here to get best IIT JEE preparation tips!!
0 notes
Link

Learn essential tips and tricks that can help you crack the JEE mains and advanced. Our experts provide study plan, important topics, mock test and tips to prepare for JEE mains. Click here to get best IIT JEE preparation tips!!
0 notes
Link

Learn essential tips and tricks that can help you crack the JEE mains and advanced. Our experts provide study plan, important topics, mock test and tips to prepare for JEE mains. Click here to get best IIT JEE preparation tips!!
0 notes