#ophthalmic technology
Text
Ophthalmic Surgical Technologies Market Size, Growth Insight
0 notes
Text
Enhancing Beauty Through Innovation: Energy-Based Aesthetic Devices Uncovered
What are Energy-Based Aesthetic Devices?
Energy-based aesthetic devices are innovative medical tools that utilize various energy sources such as laser, radiofrequency, ultrasound, and light-based technologies to perform aesthetic treatments. These devices are designed to address various skin concerns, including wrinkles, scars, pigmentation, and skin laxity, providing non-invasive or minimally…

View On WordPress
#Aesthetic Devices#Aesthetic Devices Market#Artificial Intelligence In Healthcare#Energy-Base Technology#Energy-Based Aesthetic Devices#Energy-Based Aesthetic Devices Market#Machine Learning#Medical Device#Medical Devices#Medical Devices Market#MedTech#MedTech Market#Ophthalmic Laser Devices Market
0 notes
Text
LASIK Surgery In Delhi
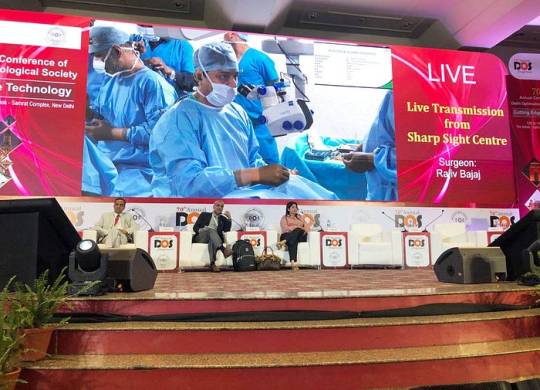
LASIK Surgery In Delhi
Dr. Rajiv Bajaj, MBBS, MS — Ophthalmology — LASIK Surgeon In Delhi
Dr. Rajiv Bajaj is a renowned Ophthalmologist practising in Pitampura area of Delhi. He is the founder of Bajaj Eye Care Centre, a centre equipped with ultra modern facilities that provides solutions to a wide range of eye ailments. The NABH accredited centre known for its professional excellence is present on the panel of leading insurance companies, Govt. Organizations and majority of TPA’s for Cashless Mediclaim Facilities Dr. Bajaj is an MBBS graduate from Maulana Azad Medical College, Delhi. He further pursued post graduation MS in Ophthalmology from Delhi University at Safdarjang Hospital which has added immensely to his surgical experience in the subject.
Prior to independent practice, Dr. Bajaj served at Safdarjang Hospital, Dr. Ram Manohar Lohia Hospital, Maharaja Agrasen Hospital and Sant Parmanand Hospital which have been his immense sources of experience in the ophthalmic field. He later established Bajaj Eye Care Centre which caters to patients from Delhi /NCR and beyond.
As a part of surgical education, Dr. Bajaj has demonstrated live surgery of MICS in national level conferences. He is among the first few doctors to incorporate advanced technology such as MICS, Phaco surgery and Refractive surgery in his practice. Dr Rajiv Bajaj has a keen interest in the field of glasses removal techniques and has always adopted the latest technology in this dimension. BAJAJ EYE CARE CENTRE takes pride to install the FIRST zeiss VISUMAX FEMTOSECOND LASER machine — SMILE for flapless, bladeless technique in the whole of NORTH Delhi.
To schedule an appointment with Dr. Rajiv Bajaj, please contact:
Name: Bajaj Eye Care Centre
Address: 101, Vikas Surya Plaza, Plot №7, DDA Community Centre Road №44, Pitampura, Delhi — 110034
Phone: 011–47024919 / 27012054
Website: www.lasikdelhi.com
You can also search for these treatments
Smile LASIK In Delhi, Smile Eye Surgery In Delhi, Smile For Spectacle Removal Delhi, Smile For Glass Removal In Delhi, LASIK Eye Surgery Center In Delhi, Laser Eye Centre In Delhi, LASIK Eye Surgeon In Delhi, LASIK Surgery Specialist In Delhi, LASIK Eye Surgery In Delhi, LASIK Surgery In Delhi, LASIK Eye Surgery Cost In Delhi, LASIK Cost In Delhi
#Smile LASIK In Delhi#Smile Eye Surgery In Delhi#Smile For Spectacle Removal Delhi#Smile For Glass Removal In Delhi#LASIK Eye Surgery Center In Delhi#Laser Eye Centre In Delhi#LASIK Eye Surgeon In Delhi#LASIK Surgery Specialist In Delhi#LASIK Eye Surgery In Delhi#LASIK Surgery In Delhi#LASIK Eye Surgery Cost In Delhi#LASIK Cost In Delhi
2 notes
·
View notes
Text

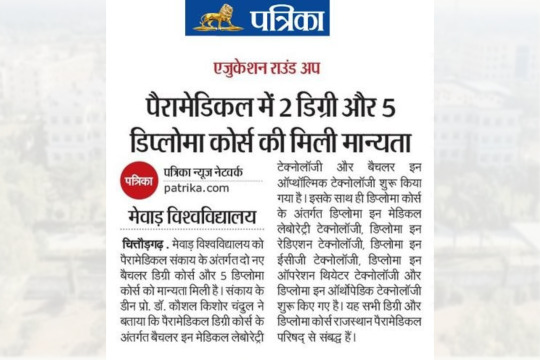







#Mewar_University_In_Media :: Recognition of two Degrees and five Diploma courses in Paramedical
7 new paramedical courses started! Two new bachelor's degree courses and five diploma courses have been recognized under the paramedical faculty at Mewar University.
👉 Admissions have started in these courses.
👉 Degree Course:
↪️ Bachelor in Medical Laboratory Technology
↪️ Bachelor in Ophthalmic Technology
👉 Diploma Course:
↪️ Diploma in Medical Laboratory Technology
↪️ Diploma in Radiation Technology
↪️ Diploma in ECG Technology
↪️ Diploma in Operation Theater Technology
↪️ Diploma in Orthopedic Technology
Published In: Rajasthan Patrika, Dainik Bhaskar, Bharat Times, Malwa Aajtak, Dainik Navajyoti, Etc.
#MewarUniversity #ParamedicalEducation #ParamedicalCourses #MedicalTraining #DiplomaPrograms #TopUniversityInRajasthan #DegreeCourses #AccreditedEducation #HealthcareCareer #News #HealthScience #BestUniversityInRajasthan #KnowledgeToWisdom #EducationUpdates
2 notes
·
View notes
Video
youtube
Could it be said that you are a Candidate for LASIK Surgery?
LASIK surgery is an extraordinary kind of ophthalmic surgery that permits people to be freed of glasses and other restorative material. The surgery includes cutting a fold of corneal tissue - , the bended, straightforward, front facing external layer of the eye - and afterward utilizing a laser to bend within the cornea to cause a change in refraction. By changing the shape of the inward cornea, the light that enters the eye is refracted in an unexpected way. For those with vision debilitations, this methodology takes into consideration restoration of vision that regularly requires contact focal points or glasses. This surgery has a high success rate ordinarily and many clients are left satisfied.
Likewise with any surgery, ophthalmologists (eye surgeons) do screen patients to check whether they are reasonable or not. In spite of the potential life-further developing advances the surgery offers, not every person can exploit these advantages and others might be excessively risky for surgery. To start with, the ophthalmologist needs to decide if the vision shortages can be amended by LASIK surgery. LASIK surgery just chips away at patients that have visual issues exclusively because of refractive mistake revised by glasses or contact focal points. Vision issues from another clinical issue isn't agreeable to adjustment through LASIK surgery. Furthermore, a few ailments might make LASIK surgery a higher risk. People with a few clinical issues, like uncontrolled diabetes and history of stroke, may not be great candidates for surgery because of their clinical issues obstructing the recuperating and success rate of surgery. People with unfortunate life expectancies are likewise not justified to be great LASIK candidates because of restricted advantage of surgery and the risk of complications during the genuine surgery. Patients likewise over a particular age may not be a candidate for LASIK too. Last, a few patients might have refractive blunder so serious that LASIK surgery won't be really useful.
A surgical assessment must be finished by an authorized ophthalmologist. Fortunately, many ophthalmologists offer free LASIK assessments for people who are keen on the surgery. This is all the more valid for ophthalmologists in confidential practice. The assessment commonly includes an intensive ophthalmology test and survey of ailments that might make one more helpless to complications during or after LASIK surgery. With propels in LASIK technology coming out like clockwork, various assessments can be made for various LASIK techniques with benefits and downsides redid for every individual assessed. The ophthalmologist will then provide the individual with a decision of regardless of whether to have the surgery, after the risks and advantages have been made sense of. LASIK surgery is considered cosmetic, so some insurance organizations may not pay everything for surgery.
2 notes
·
View notes
Text
Benefits of LASIK surgery

LASIK ophthalmic therapy is vision correction surgery where it reshapes the call of treating loss. , Electric Simply put, it is a refractive surgery beneficial for hyperopia (farsightedness), and myopia (nearsightedness), and is particularly helpful in correcting astigmatism as it is in the cornea. In addition, the US Drug Administration (FDA) approved it in 1999 and tied it to doctors who want to get rid of glasses or improve their vision. Do LASIK Eye Surgeries .
Nowadays, old people, youth and even children are facing low vision and other eye related problems. Is no one other than the one with glasses can understand the freedom to get only through glasses. , Many wear contact lenses for the rest of the day by wearing glasses or spectacles that affect their outer arms but also their.
Advanced technology will let you get completely rid of contact lenses through LASIK surgery and brand Lasik Surgery.
Mostly it happens that it takes at least 15 minutes on lasik on both eyes. The treatment of this surgery is painless.
In most cases, it may take a few minutes to perform LASIK surgery on both eyes. It is non-treatment and provides better vision to the patient. Most of the patients also too, it provides a permanent solution to surge surgery lens lenses or glasses.
Complications in all types of surgeries Complications, risks and side effects. , But LASIK one of the safest elective surgeries because it has a rate of complications. , This permission is because it is never the cause of blindness or blindness. Ok. In addition, this surgery is the more studied alternative Lasik.
Benefits of LASIK surgery
Lasik sir pray. Now people can get rid of spectacles and improve their visual impairment through this surgery. , Lasik surgery will be worth it because of its benefits:
Tatka Razor Once you do the LASIK surgery, the very next moment the surgery will be completely your desired vision and you will be able to taste it immediately.
Approx Rate Pray This surge is associated with little or no pain which helps to heal with desired results. , is is
Permanent results - Most people live about the long-term effects because they think they have. , have have have have have have have have have have have have those uto shal yija hai hai hai can… hai hai s th nija. But the surgery provides permanent benefits and in future you will not have to worry about the eyesight.
Easy and Recovery - Not only is the process of surgery smooth but its recovery most important thing is that people may not face any serious problems or difficulties in future No complications will be observed after the surgery. It is very fast and easy like magic because just 15 mins surgery can give you clear vision for a long time.. , The only thing you will have to deal with for months would be very mild tearing a slight burning in the eyes which can be easily rectified.
you can contact best eye care hospital for Lasik Eye Surgery.
Satisfaction - Lasik surgery c keep porting a c keep going.
Better vision and vision - LASIK surgery will help in the treatment of eyes which will not make you want and vision without any Save money and follow. Once a person undergoes this surgery, then he/she can buy eyewear like glasses, contact lenses, etc.
2 notes
·
View notes
Text
HuidaGene and Synthego Announce Licensing Agreement for Next-Generation Gene Editing Enzyme hfCas12Max, Business News
https://www.merchant-business.com/huidagene-and-synthego-announce-licensing-agreement-for-next-generation-gene-editing-enzyme-hfcas12max-business-news/?feed_id=7497&_unique_id=665a092c6068b
HuidaGene and Synthego Announce Licensing Agreement for Next-Generation Gene Editing Enzyme hfCas12Max, Business News
Business
SAN FRANCISCO, Nov. 2, 2017 /PRNewswire/ — HuidaGene Therapeutics (“HuidaGene”), a global clinical-stage biotechnology company focused on the development of CRISPR-based genomic medicines, and Synthego Corporation (“Synthego”), a leading provider of innovative CRISPR solutions for cell and gene therapy development, are proud to announce a collaborative license agreement for the high-fidelity Cas12 CRISPR nuclease (hfCas12Max). The collaboration highlights the significant clinical utility of hfCas12Max and naturally complements Synthego’s focus on advanced GMP manufacturing capabilities. Under the license agreement and undisclosed financial terms between HuidaGene and Synthego, HuidaGene will grant Synthego the rights to manufacture and commercialize hfCas12Max nuclease and research-optimized gRNAs. HuidaGene will also grant Synthego the right to sublicense the nuclease for therapeutic uses.
The collaboration will streamline the development of CRISPR-based therapeutic applications, providing developers access to highly precise and efficient next-generation genome editing tools. Xuan YaoHuidaGene Co-Founder and President, Ph.D., highlighted the importance of the agreement, saying, “HuidaGene’s extensive CRISPR intellectual property portfolio sets us apart as a pioneer in genomic medicine with a versatile pipeline targeting significant neurological and ophthalmic diseases. By working with Synthego, we are poised to significantly accelerate the advancement of CRISPR-based therapeutics and deliver life-changing genomic medicines to patients around the world.”
Craig ChristiansonThe CEO of Synthego expressed enthusiasm about the collaboration with HuidaGene on hfCas12Max, stating: “Combining our advanced CRISPR GMP production capabilities and regulatory expertise with HuidaGene’s next-generation nuclease technology is a critical step in advancing innovative cell and gene therapies. This collaboration highlights Synthego’s commitment to CRISPR-based therapeutics, strengthened by focused investments in GMP manufacturing and comprehensive CRISPR solutions supporting therapeutic development. The integration of hfCas12Max is a natural fit with our efforts to increase the accessibility and efficiency of CRISPR tools.”
hfCas12Max is a product of HuidaGene. HG-Precision This platform stands out for its excellent on-target editing efficiency and reduced off-target editing activity in mammalian cells. This novel CRISPR gene editing system also offers the advantage of packaging into a single viral vector, a key requirement for many cell and gene therapies. The commercialization of hfCas12Max will greatly increase the accessibility of this CRISPR gene editing system, providing greater operational freedom and facilitating the development of CRISPR-based cell and gene therapies.
The collaboration strengthens both companies’ positions as catalysts for innovation and advances their shared mission to transform genome-based cell and gene therapy to deliver unprecedented therapeutic outcomes.
For partnership inquiries, please contact inquiry and inquiry.
hfCas12Max is a registered trademark. China.
About HuidaGene
HuidaGene Therapeutics is a proprietary CRISPR-based HG-Precision®A platform for discovering, designing and developing potentially curative genomic medicines. The company is advancing its HG004 clinical program. RPE65CRISPR RNA editing in inherited retinal diseases (conferring both ODD and RPDD), HG202 neovascular age-related macular degeneration, and preclinical pipeline (including CRISPR RNA editing in
HG204 neurodevelopmental disease) Mekup 2 overlap syndrome (received both ODD and RPDD from US FDA and both ODD from EMA), HG302 CRISPR DNA editing for Duchenne muscular dystrophy (received both ODD and RPDD), and HG303 CRISPR DNA editing for amyotrophic lateral sclerosis (ALS). Our extensive intellectual property portfolio positions us as a leader in realizing the full potential of genomic medicine. For more information, huidagene.com or LinkedIn.
About Synthego
Synthego is a pioneering provider of genome engineering solutions, offering comprehensive products and services to accelerate the development of CRISPR-based cell and gene therapies. With a commitment to empowering researchers and innovators, Synthego’s cutting-edge CRISPR technology and expertise drive advancements from preclinical to clinical applications. For more information, please visit Shinsegoor LinkedIn.
Source of this program
“The components are amazing and I’m hooked!!”
“SHANGHAI and REDWOOD CITY, Calif., May 29, 2024 /PRNewswire/ — HuidaGene Therapeutics (“HuidaGene”), a global clinical-stage biotechnology company focused on the development of CRISPR-based genomic medicines, and…”
Source: Read more
Source link: https://www.asiaone.com/business/huidagene-and-synthego-announce-licensing-agreement-next-generation-gene-editing-enzyme
The post HuidaGene and Synthego Announce Licensing Agreement for Next-Generation Gene Editing Enzyme hfCas12Max, Business News appeared first on Merchant Business News.
Buy, Sell, Get Informed, Negotiate and Be Happy!
Business, Global
0 notes
Text
HuidaGene and Synthego Announce Licensing Agreement for Next-Generation Gene Editing Enzyme hfCas12Max, Business News - #Business #Global
https://www.merchant-business.com/huidagene-and-synthego-announce-licensing-agreement-for-next-generation-gene-editing-enzyme-hfcas12max-business-news/?feed_id=7496&_unique_id=665a092b4b406
HuidaGene and Synthego Announce Licensing Agreement for Next-Generation Gene Editing Enzyme hfCas12Max, Business News
Business
SAN FRANCISCO, Nov. 2, 2017 /PRNewswire/ — HuidaGene Therapeutics (“HuidaGene”), a global clinical-stage biotechnology company focused on the development of CRISPR-based genomic medicines, and Synthego Corporation (“Synthego”), a leading provider of innovative CRISPR solutions for cell and gene therapy development, are proud to announce a collaborative license agreement for the high-fidelity Cas12 CRISPR nuclease (hfCas12Max). The collaboration highlights the significant clinical utility of hfCas12Max and naturally complements Synthego’s focus on advanced GMP manufacturing capabilities. Under the license agreement and undisclosed financial terms between HuidaGene and Synthego, HuidaGene will grant Synthego the rights to manufacture and commercialize hfCas12Max nuclease and research-optimized gRNAs. HuidaGene will also grant Synthego the right to sublicense the nuclease for therapeutic uses.
The collaboration will streamline the development of CRISPR-based therapeutic applications, providing developers access to highly precise and efficient next-generation genome editing tools. Xuan YaoHuidaGene Co-Founder and President, Ph.D., highlighted the importance of the agreement, saying, “HuidaGene’s extensive CRISPR intellectual property portfolio sets us apart as a pioneer in genomic medicine with a versatile pipeline targeting significant neurological and ophthalmic diseases. By working with Synthego, we are poised to significantly accelerate the advancement of CRISPR-based therapeutics and deliver life-changing genomic medicines to patients around the world.”
Craig ChristiansonThe CEO of Synthego expressed enthusiasm about the collaboration with HuidaGene on hfCas12Max, stating: “Combining our advanced CRISPR GMP production capabilities and regulatory expertise with HuidaGene’s next-generation nuclease technology is a critical step in advancing innovative cell and gene therapies. This collaboration highlights Synthego’s commitment to CRISPR-based therapeutics, strengthened by focused investments in GMP manufacturing and comprehensive CRISPR solutions supporting therapeutic development. The integration of hfCas12Max is a natural fit with our efforts to increase the accessibility and efficiency of CRISPR tools.”
hfCas12Max is a product of HuidaGene. HG-Precision This platform stands out for its excellent on-target editing efficiency and reduced off-target editing activity in mammalian cells. This novel CRISPR gene editing system also offers the advantage of packaging into a single viral vector, a key requirement for many cell and gene therapies. The commercialization of hfCas12Max will greatly increase the accessibility of this CRISPR gene editing system, providing greater operational freedom and facilitating the development of CRISPR-based cell and gene therapies.
The collaboration strengthens both companies’ positions as catalysts for innovation and advances their shared mission to transform genome-based cell and gene therapy to deliver unprecedented therapeutic outcomes.
For partnership inquiries, please contact inquiry and inquiry.
hfCas12Max is a registered trademark. China.
About HuidaGene
HuidaGene Therapeutics is a proprietary CRISPR-based HG-Precision®A platform for discovering, designing and developing potentially curative genomic medicines. The company is advancing its HG004 clinical program. RPE65CRISPR RNA editing in inherited retinal diseases (conferring both ODD and RPDD), HG202 neovascular age-related macular degeneration, and preclinical pipeline (including
CRISPR RNA editing in HG204 neurodevelopmental disease) Mekup 2 overlap syndrome (received both ODD and RPDD from US FDA and both ODD from EMA), HG302 CRISPR DNA editing for Duchenne muscular dystrophy (received both ODD and RPDD), and HG303 CRISPR DNA editing for amyotrophic lateral sclerosis (ALS). Our extensive intellectual property portfolio positions us as a leader in realizing the full potential of genomic medicine. For more information, huidagene.com or LinkedIn.
About Synthego
Synthego is a pioneering provider of genome engineering solutions, offering comprehensive products and services to accelerate the development of CRISPR-based cell and gene therapies. With a commitment to empowering researchers and innovators, Synthego’s cutting-edge CRISPR technology and expertise drive advancements from preclinical to clinical applications. For more information, please visit Shinsegoor LinkedIn.
Source of this program
“The components are amazing and I’m hooked!!”
“SHANGHAI and REDWOOD CITY, Calif., May 29, 2024 /PRNewswire/ — HuidaGene Therapeutics (“HuidaGene”), a global clinical-stage biotechnology company focused on the development of CRISPR-based genomic medicines, and…”
Source: Read more
Source link: https://www.asiaone.com/business/huidagene-and-synthego-announce-licensing-agreement-next-generation-gene-editing-enzyme
The post HuidaGene and Synthego Announce Licensing Agreement for Next-Generation Gene Editing Enzyme hfCas12Max, Business News appeared first on Merchant Business News.
Buy, Sell, Get Informed, Negotiate and Be Happy!
Business, Global
BLOGGER - #Business #Global
0 notes
Text
HuidaGene and Synthego Announce Licensing Agreement for Next-Generation Gene Editing Enzyme hfCas12Max, Business News
https://www.merchant-business.com/huidagene-and-synthego-announce-licensing-agreement-for-next-generation-gene-editing-enzyme-hfcas12max-business-news/?feed_id=7495&_unique_id=665a092903c5a
HuidaGene and Synthego Announce Licensing Agreement for Next-Generation Gene Editing Enzyme hfCas12Max, Business News
Business
SAN FRANCISCO, Nov. 2, 2017 /PRNewswire/ — HuidaGene Therapeutics (“HuidaGene”), a global clinical-stage biotechnology company focused on the development of CRISPR-based genomic medicines, and Synthego Corporation (“Synthego”), a leading provider of innovative CRISPR solutions for cell and gene therapy development, are proud to announce a collaborative license agreement for the high-fidelity Cas12 CRISPR nuclease (hfCas12Max). The collaboration highlights the significant clinical utility of hfCas12Max and naturally complements Synthego’s focus on advanced GMP manufacturing capabilities. Under the license agreement and undisclosed financial terms between HuidaGene and Synthego, HuidaGene will grant Synthego the rights to manufacture and commercialize hfCas12Max nuclease and research-optimized gRNAs. HuidaGene will also grant Synthego the right to sublicense the nuclease for therapeutic uses.
The collaboration will streamline the development of CRISPR-based therapeutic applications, providing developers access to highly precise and efficient next-generation genome editing tools. Xuan YaoHuidaGene Co-Founder and President, Ph.D., highlighted the importance of the agreement, saying, “HuidaGene’s extensive CRISPR intellectual property portfolio sets us apart as a pioneer in genomic medicine with a versatile pipeline targeting significant neurological and ophthalmic diseases. By working with Synthego, we are poised to significantly accelerate the advancement of CRISPR-based therapeutics and deliver life-changing genomic medicines to patients around the world.”
Craig ChristiansonThe CEO of Synthego expressed enthusiasm about the collaboration with HuidaGene on hfCas12Max, stating: “Combining our advanced CRISPR GMP production capabilities and regulatory expertise with HuidaGene’s next-generation nuclease technology is a critical step in advancing innovative cell and gene therapies. This collaboration highlights Synthego’s commitment to CRISPR-based therapeutics, strengthened by focused investments in GMP manufacturing and comprehensive CRISPR solutions supporting therapeutic development. The integration of hfCas12Max is a natural fit with our efforts to increase the accessibility and efficiency of CRISPR tools.”
hfCas12Max is a product of HuidaGene. HG-Precision This platform stands out for its excellent on-target editing efficiency and reduced off-target editing activity in mammalian cells. This novel CRISPR gene editing system also offers the advantage of packaging into a single viral vector, a key requirement for many cell and gene therapies. The commercialization of hfCas12Max will greatly increase the accessibility of this CRISPR gene editing system, providing greater operational freedom and facilitating the development of CRISPR-based cell and gene therapies.
The collaboration strengthens both companies’ positions as catalysts for innovation and advances their shared mission to transform genome-based cell and gene therapy to deliver unprecedented therapeutic outcomes.
For partnership inquiries, please contact inquiry and inquiry.
hfCas12Max is a registered trademark. China.
About HuidaGene
HuidaGene Therapeutics is a proprietary CRISPR-based HG-Precision®A platform for discovering, designing and developing potentially curative genomic medicines. The company is advancing its HG004 clinical program. RPE65CRISPR RNA editing in inherited retinal diseases (conferring both ODD and RPDD), HG202 neovascular age-related macular degeneration, and preclinical pipeline (including CRISPR RNA editing in
HG204 neurodevelopmental disease) Mekup 2 overlap syndrome (received both ODD and RPDD from US FDA and both ODD from EMA), HG302 CRISPR DNA editing for Duchenne muscular dystrophy (received both ODD and RPDD), and HG303 CRISPR DNA editing for amyotrophic lateral sclerosis (ALS). Our extensive intellectual property portfolio positions us as a leader in realizing the full potential of genomic medicine. For more information, huidagene.com or LinkedIn.
About Synthego
Synthego is a pioneering provider of genome engineering solutions, offering comprehensive products and services to accelerate the development of CRISPR-based cell and gene therapies. With a commitment to empowering researchers and innovators, Synthego’s cutting-edge CRISPR technology and expertise drive advancements from preclinical to clinical applications. For more information, please visit Shinsegoor LinkedIn.
Source of this program
“The components are amazing and I’m hooked!!”
“SHANGHAI and REDWOOD CITY, Calif., May 29, 2024 /PRNewswire/ — HuidaGene Therapeutics (“HuidaGene”), a global clinical-stage biotechnology company focused on the development of CRISPR-based genomic medicines, and…”
Source: Read more
Source link: https://www.asiaone.com/business/huidagene-and-synthego-announce-licensing-agreement-next-generation-gene-editing-enzyme
The post HuidaGene and Synthego Announce Licensing Agreement for Next-Generation Gene Editing Enzyme hfCas12Max, Business News appeared first on Merchant Business News.
Buy, Sell, Get Informed, Negotiate and Be Happy!
0 notes
Text
Transforming Beauty: The Power of Energy-Based Aesthetic Devices
What are Energy-Based Aesthetic Devices?
Energy-based aesthetic devices are innovative medical tools that utilize various energy sources such as laser, radiofrequency, ultrasound, and light-based technologies to perform aesthetic treatments. These devices are designed to address various skin concerns, including wrinkles, scars, pigmentation, and skin laxity, providing non-invasive or minimally…

View On WordPress
#Aesthetic Devices#Aesthetic Devices Market#Artificial Intelligence In Healthcare#Energy-Base Technology#Energy-Based Aesthetic Devices#Energy-Based Aesthetic Devices Market#Machine Learning#Medical Device#Medical Devices#Medical Devices Market#MedTech#MedTech Market#Ophthalmic Laser Devices Market
0 notes
Text
Eye-Q Hospital in Bhiwani: Revolutionizing Eye Care in India
Eye-Q Hospital in Bhiwani stands as a beacon of excellence in the field of ophthalmology. As part of the esteemed Eye-Q India network, the Bhiwani branch upholds the highest standards of eye care, ensuring that patients receive top-notch services and treatments.
Comprehensive Eye Care Services
Eye-Q Hospital in Bhiwani offers a wide range of eye care services, catering to diverse needs. These include routine eye examinations, advanced cataract surgery, LASIK and refractive surgery, glaucoma treatment, pediatric ophthalmology, and retina services. Each service is delivered by a team of highly skilled ophthalmologists and support staff, equipped with state-of-the-art technology.
Cutting-Edge Technology
The hospital is renowned for its use of cutting-edge technology in diagnosing and treating eye conditions. Eye-Q Bhiwani employs advanced equipment such as OCT (Optical Coherence Tomography), YAG laser, and phacoemulsification machines, ensuring precise and effective treatments. This commitment to technological advancement sets Eye-Q apart from other eye care providers in the region.
Skilled and Experienced Team
The team at Eye-Q Hospital in Bhiwani comprises experienced ophthalmologists, optometrists, and support staff who are dedicated to providing exceptional care. Their expertise spans various sub-specialties in ophthalmology, ensuring comprehensive care for all types of eye conditions. Regular training and workshops keep the team updated with the latest advancements in the field.
Patient-Centric Approach
Eye-Q Bhiwani prides itself on a patient-centric approach. The hospital is designed to provide a comfortable and welcoming environment, with a focus on personalized care. Each patient's treatment plan is tailored to their specific needs, ensuring the best possible outcomes. This approach has earned the hospital a high level of trust and satisfaction among its patients.
Community Outreach and Education
Eye-Q Hospital in Bhiwani is also actively involved in community outreach and education programs. These initiatives aim to raise awareness about eye health and provide free or subsidized eye care services to underprivileged communities. Through regular eye camps, school screenings, and public seminars, Eye-Q Bhiwani is making a significant impact on public health in the region.
Part of the Eye-Q India Network
As part of the Eye-Q India network, the Bhiwani hospital benefits from shared resources, expertise, and best practices from across the country. Eye-Q India is a leading eye care provider with a network of hospitals and clinics dedicated to excellence in eye care. This affiliation ensures that Eye-Q Bhiwani maintains high standards and stays at the forefront of ophthalmic care in India.
Conclusion
Eye-Q Hospital in Bhiwani is a premier institution for eye care, offering a comprehensive range of services with a patient-centric approach. Its use of advanced technology, skilled team, and community-focused initiatives make it a standout choice for anyone seeking quality eye care in the region. As part of the Eye-Q India network, Eye-Q Bhiwani continues to set benchmarks in the field of ophthalmology, ensuring that patients receive the best possible care.
0 notes
Text
A Market on the Move: Exploring Investment Opportunities in Global Refractive surgery device Market
The global refractive surgery devices market is set to experience steady growth, with market valuation anticipated to reach USD 213.0 million by the year 2024. According to recent industry projections, the market is expected to grow at a compound annual growth rate (CAGR) of 3.90% from 2024 to 2034, ultimately offering an absolute dollar opportunity of USD 312.3 million by the end of the forecast period.
Request Your Detailed Report Sample With Your Work Email:
https://www.futuremarketinsights.com/reports/sample/rep-gb-780
Key Takeaways:
Market Valuation: The refractive surgery device market is projected to reach a valuation of US$ 213.0 million by 2024.
Growth Rate: The market is expected to expand at a CAGR of 3.90% from 2024 to 2034.
Future Opportunity: By the end of 2034, the market is anticipated to offer an absolute dollar opportunity of US$ 312.3 million.
Driving Factors: Key factors fueling this growth include an increasing elderly population and the rise in screen time due to Covid-19 restrictions, leading to a higher prevalence of vision issues requiring correction.
Competitive Landscape:
Owing to its extended existence, the market for equipment used in refractive surgery is crowded with businesses that have established a solid presence abroad. The devices needed for refractive procedures are supplied by these firms to hospitals, ambulatory surgical centers, and emergency clinics.
Alcon Inc., Carl Zeiss Meditec AG, Johnson & Johnson Vision, Bausch + Lomb, NIDEK Co., Ltd., and Ziemer Ophthalmic Systems AG are a few of the leading businesses in the industry. These businesses spend billions of dollars developing innovative technology to streamline, expedite, and improve the quality of surgery.
Recent Developments
In June 2023, Eye-Q introduced an Advanced Customised LASIK machine at its Rewari eye-care facility, revolutionizing laser vision correction and eliminating the need for patients to travel to Gurugram. The hospital aimed to enhance the quality of life for individuals, particularly youth aged 18-35, by offering world-class eye-care treatments.
In September 2023, Alcon unveiled its largest-ever scientific program at the 41st Congress of the ESCRS in Vienna, Austria. The event showcased over 75 abstracts, presenting new data and innovations supporting ophthalmic surgical teams across cataract, refractive, visualization, and ocular health fields.
Key Companies in the Market:
Alcon Inc.
Carl Zeiss Meditec AG
Johnson & Johnson Vision
Bausch + Lomb
NIDEK Co., Ltd.
Ziemer Ophthalmic Systems AG
STAAR Surgical Company
Abbott Medical Optics
Topcon Corporation
Essilor International
Lumenis Ltd.
HOYA Corporation
Haag-Streit AG
Schwind eye-tech-solutions GmbH
iVIS Technologies
Oculentis GmbH
NIDEK Technologies Srl
Optovue, Inc.
OCULUS Optikgeräte GmbH
Rayner Intraocular Lenses Limited
Refractive Surgery Device Market Segmentation:
By Product Type:
Lasers
Microkeratome
Aberrometers
Other Product Types
By Application:
Myopia
Hyperopia
Astigmatism
Presbyopia
Dry Eyes
By End-use:
Hospitals
Ambulatory Surgery Centers
Ophthalmology Clinics
By Region:
North America
Latin America
Asia Pacific
Middle East and Africa (MEA)
Europe
0 notes
Text
Comprehensive Guide to LASIK and Phaco Surgery: What Aspiring Ophthalmologists Need to Know
As an aspiring ophthalmologist, it's essential to have a deep understanding of two of the most common and transformative procedures in the field: LASIK and phacoemulsification (phaco) surgery. These procedures not only have the potential to restore vision and improve quality of life for countless patients but also represent the pinnacle of technological advancements and surgical precision in ophthalmology.
Lasik Training in India | Lasik Laser In Kanpur | Lasik Surgery In kanpur
LASIK: Reshaping the Future of Vision Correction LASIK, short for Laser-Assisted In Situ Keratomileusis, is a revolutionary refractive surgery that has transformed the way we approach vision correction. This procedure involves the use of an excimer laser to precisely reshape the cornea, the transparent front part of the eye, to correct refractive errors such as nearsightedness, farsightedness, and astigmatism.
During the LASIK procedure, a thin flap is created on the cornea using a precise microkeratome or femtosecond laser. The excimer laser then removes microscopic amounts of corneal tissue, reshaping the curvature to achieve the desired refractive correction. The flap is then repositioned, allowing the cornea to heal and the vision to improve rapidly.
Phaco Training | Phaco Training In India
Mastering LASIK requires a deep understanding of corneal anatomy, refractive principles, and laser physics. It also demands exceptional surgical skills, meticulous attention to detail, and a commitment to ongoing education and training to stay abreast of the latest technological advancements in the field.
Phacoemulsification: Restoring Clarity with Precision Phacoemulsification, or phaco, is a revolutionary technique for removing cataracts, a clouding of the eye's natural lens that can significantly impair vision. This minimally invasive procedure involves the use of ultrasonic energy to break up and remove the clouded lens material, followed by the implantation of an artificial intraocular lens (IOL) to restore clear vision.
During phaco surgery, a small incision is made in the cornea, and a specialized ultrasonic probe is inserted into the eye. This probe emits high-frequency sound waves that break up the cataract into tiny pieces, which are then gently suctioned out of the eye. Once the cataract has been removed, a foldable IOL is carefully inserted through the same incision and positioned in the capsular bag, the natural lens capsule.
Mastering phaco surgery requires a deep understanding of lens anatomy, cataract pathophysiology, and the principles of ultrasound and fluidics. It also demands exceptional surgical dexterity, precise wound construction, and the ability to manage potential complications with confidence and expertise.
Lasik Training in India

The Importance of Mastering Both Techniques For aspiring ophthalmologists, mastering both LASIK and phaco surgery is crucial for providing comprehensive, state-of-the-art care to patients. These procedures not only address different visual concerns but also cater to different patient populations and age groups.
LASIK is primarily sought by younger patients seeking to reduce or eliminate their dependence on glasses or contact lenses, while phaco surgery is more commonly performed on older patients with age-related cataracts. By becoming proficient in both techniques, ophthalmologists can offer a wide range of vision correction solutions tailored to each patient's unique needs and preferences.
Mahendra Eye Institute | Eye Hospital in Kanpur
Furthermore, the skills and principles learned through mastering these procedures are invaluable for other areas of ophthalmic practice, such as anterior segment surgery, corneal transplantation, and the management of complex cases involving multiple vision-impairing conditions.
In conclusion, LASIK and phaco surgery represent the cutting edge of ophthalmology, offering life-changing vision restoration and correction for millions of patients worldwide. As an aspiring ophthalmologist, dedicating time and effort to mastering these techniques will not only expand your surgical repertoire but also position you as a highly skilled and versatile practitioner capable of providing exceptional care to a diverse range of patients.
Lasik Laser eye specialist in Kanpur | Remove Eye Glasses | Best Lasik Eye Surgery in Kanpur
#phaco training#phaco training in india#kanpur#mahendra eye institute#eye hospital in kanpur#lasik training in india#lasik treatment#lasik eye surgery
0 notes
Text
Surgical Sutures Market to be Worth $4.42 Billion by 2030
Meticulous Research®, a prominent firm in market research, has released a report titled, ‘Surgical Sutures Market by Type [Absorbable {Polyglactin, Polydioxanone, Poliglecaprone, Collagen} Nonabsorbable {Polypropylene, Nylon, Silk}] Structure [Monofilament, Multifilament] Coating [Uncoated, Antimicrobial] Application - Global Forecast to 2030.’
As per the recent report by Meticulous Research®, the surgical sutures market is anticipated to achieve a value of $4.42 billion by 2030, with a compound annual growth rate (CAGR) of 4.1% expected from 2023 to 2030. The market's expansion is fueled by advancements in suture and wound closure technologies, alongside a rise in surgical procedures. Moreover, the improving healthcare infrastructure in emerging economies presents promising avenues for market growth. However, the emergence of alternative wound care solutions and the increasing preference for minimally invasive surgeries are likely to moderately impede market growth.
Surgical Sutures Market: Future Outlook
The surgical sutures market is categorized based on various factors including Type [Absorbable Sutures {Synthetic Sutures (Polyglactin 910 (Vicryl), Poliglecaprone 25 (MONOCRYL), Polydioxanone (PDS), Polyglycolic Acid), Natural Sutures (Collagen Fiber Suture), Other Absorbable Sutures}, Nonabsorbable Sutures {Synthetic Sutures (ETHIBOND EXCEL Polyester Suture, Polypropylene (Prolene)), Natural Sutures (Nylon (polyamide), Silk, Stainless Steel), Other Nonabsorbable Sutures}, Structure [Monofilament Sutures, Multifilament/Braided Sutures], Coating Type [Uncoated Sutures, Antimicrobial Sutures], Application [Cardiovascular Surgery, Orthopedic Surgery, Neurosurgery, Gynecological Surgery, Cosmetic & Plastic Surgery, Ophthalmic Surgery, General Surgery, Other Applications], End User (Hospitals/Clinics, Ambulatory Surgical Centers, Other End Users], and Geography (North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa).
Based on the type, in 2023, the absorbable sutures category is projected to dominate the surgical sutures market, primarily due to advancements in suture technology and their increased tensile strength. These sutures are commonly employed for deep wound closure and offer anti-inflammatory properties, eliminating the need for removal and reducing additional trauma to the wound site.
Based on structure, by 2023, it is anticipated that the multifilament/braided sutures segment will hold the leading position in the surgical sutures market. These sutures are crafted from twisted filaments, offering notable strength and flexibility. Thanks to their higher friction coefficient, multifilament suture materials typically exhibit impressive strength and ensure secure knotting.
Based on the coating type, in 2023, the antimicrobial sutures segment is expected to experience the most significant compound annual growth rate (CAGR) throughout the forecast period. This growth is propelled by the imperative to mitigate surgical site infections, a surge in surgical interventions, favorable reimbursement schemes, and the expanding elderly population.
Based on application, the surgical sutures market is divided into various applications, including cardiovascular surgery, orthopedics, neurosurgery, gynecological surgery, cosmetic and plastic surgery, ophthalmic surgery, general surgery, and other uses. Among these, the orthopedic surgery segment is projected to hold the largest portion of the market. This dominance is fueled by factors such as the growing incidence of musculoskeletal conditions, the uptick in associated surgical interventions, the aging population, and the increasing uptake of advanced surgical techniques.
Based on end user, in 2023, it is projected that the hospitals/clinics segment will hold the biggest portion of the surgical sutures market. This dominance is attributed to an increase in patient visits to hospitals, driven by higher healthcare spending and a rise in the number of hospitals.
Based on geography, by 2023, it is anticipated that North America will hold the dominant share of the surgical sutures market. This is primarily due to the presence of numerous key market players, a significant volume of surgical procedures, widespread adoption of advanced suturing technologies, and well-developed healthcare systems across the region.
Key Players:
The surgical sutures market report profiles several key players, including Ethicon, Inc. (U.S.) (A subsidiary of Johnson & Johnson Services, Inc.), B. Braun SE (Germany), Medtronic (Ireland), Smith & Nephew plc (U.K.), Surgical Specialties Corporation (U.S.), CONMED Corporation (U.S.), Mellon Medical B.V. (Netherlands), DemeTECH Corporation (U.S.), Apollo Endosurgery, Inc. (U.S.), Teleflex Incorporated (U.S.), Kono Seisakusho Co.,Ltd. (Japan), Zimmer Biomet Holdings (U.S.), Stryker Corporation (U.S.), Boston Scientific Corporation (U.S.), Advanced MedTech Solutions Pvt. Ltd. (India), and Peters Surgical (India).
Download Research Sample @ https://www.meticulousresearch.com/download-sample-report/cp_id=4947
Key questions answered in the report-
What are the market segments showing notable growth potential in terms of type, structure, coating type, application, end user, and geography?
What was the past performance of the global surgical sutures market?
What are the projected market figures and estimations for the timeframe spanning from 2023 to 2030?
What are the primary factors propelling, inhibiting, and presenting opportunities within the surgical sutures market?
Who are the prominent participants in the surgical sutures market?
What does the competitive scenario look like, and which companies are leading the surgical sutures market?
What are the latest advancements or updates in the surgical sutures market?
What tactics do the key players in the surgical sutures market use to stay ahead?
What are the trends in different geographical regions and which regions?
Contact Us:
Meticulous Research®
Email- [email protected]
Contact Sales- +1-646-781-8004
Connect with us on LinkedIn- https://www.linkedin.com/company/meticulous-research
#Surgical Sutures Market#Surgical Stitches#Absorbable Sutures#Surgical threads#Synthetic sutures#Natural sutures
0 notes
Text
Best Eye Hospital in Siliguri
Looking for the best eye hospital in Siliguri? Choose our top facility for ophthalmic care with quality of technology. For consultation visit: https://www.desunsiliguri.com/ophthalmology-eye/
0 notes
Text
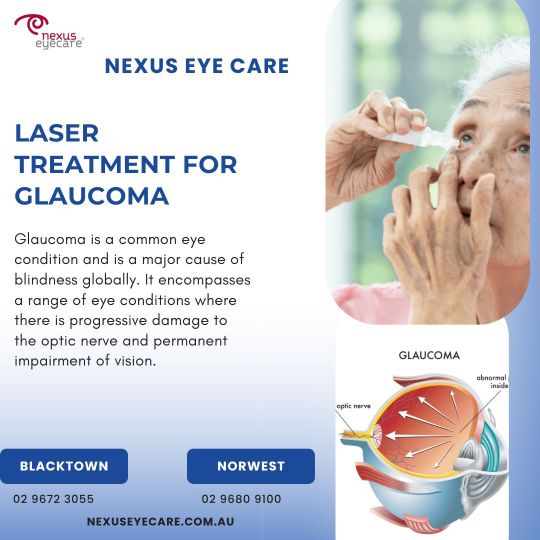
Laser Treatment for Glaucoma - Blacktown and Norwest
At Nexus Eye Care, we specialize in advanced laser treatment for glaucoma, providing effective solutions to manage this eye condition. Our expert team utilizes the latest laser technology to reduce intraocular pressure, helping to prevent vision loss and improve your quality of life. Learn more about our comprehensive glaucoma care and how our personalized treatment plans can benefit you. Visit Nexus Eye Care for top-tier ophthalmic services tailored to your needs.
#glaucoma#glaucoma specialist#glaucoma treatment#ophthalmology#blacktown#eye care#norwest#Nexuseyecare
0 notes