#d&dmap
Text
Here's what I've been up to!
Are people returning to tumblr?? That would make me so happy!
I've been doing a lot for the past couple years! Aside from my career as a tattoo artist, I've been doing a lot of art of Dungeons and Dragons, and after drawing fanart and commissions for d&d for years, I finally started playing! One of the major things I've started doing is drawing map and token assets that can be used for digital tabletops, like roll20. I do top down assets and lots of bust-style tokens, but my favourite thing to do has been isometric assets.
My maps and tokens are for sale on roll20 and patreon, and I've also got a tiktok where you can see them in motion and find out how to use them in your game! It's really not as complicated as it looks, and I find the isometric maps add a lot to my game.
I also have some plothooks, npcs, villians, and traps on my tiktok and patreon that you can use in your own game, and you can learn about my magical girls/horror home game.
Here are a few examples of maps and tokens I've done!





#dnd map#dungeons and dragons#dnd#d&d#d&dmap#isometric map#isometric art#dnd art#dnd5e#dnd stuff#dnd maps#dnd mapmaker#dnd monster#dungeons and dragons maps#dungeons and dragons art#dungeons and dragons tokens#roll20#ttrpg#digital ttrpg
173 notes
·
View notes
Text

Moated Jungle Keep 3500x3500
Who rules these ruins?
#Dungeons & Dragons#D&D#Map#Maps#D&Dmaps#5e#5th edition#homebrew#roll20#Jungle maps#jungle#ttrpg#rpg#DnD#Dungeons and Dragons#Dungeon Master#Chult#Keep#Castle ruins#Moat#Castle maps
17 notes
·
View notes
Text
3 Tips to Grow Your Business With a Digital Marketing Agency
Are you interested in starting a digital marketing agency in Lahore but aren't sure how to proceed? Read this article and discover 3 tips to grow your business with a digital marketing agency. The first tip is to focus on a niche and focus on providing value-added services. The second tip is to get your blog posts published on other digital marketing agency websites. This way, you'll be able to attract a broader audience and grow your agency's revenue.
Growing a digital marketing agency in Lahore
Building a digital marketing agency is crucial to the success of your company. The internet is where most people start their search, so if your business doesn't have a strong digital presence, you're missing out on a huge market. While it won't happen overnight, building your own digital marketing agency in Lahore will save you both time and money. There are several steps you can take to improve the overall performance of your business.
One option for growing your business with a digital marketing agency is to outsource certain tasks that require expertise. Consider creating webinars and collaborating with other agencies and industry influencers. Guest posting on industry blogs and creating affiliate programs are two effective ways to reach influential people. These are just a few of the many ways to grow your business. In addition, if you're just starting out, you may want to consider hiring marketing experts to work with you on the most important tasks.
When choosing a digital marketing agency, consider the size of your business. A digital marketing agency that has several years of experience can offer more than one service. Choosing one with a large staff may not be a good idea. You may have to work with multiple agencies at the same time to ensure you get the right combination of services. One of the best ways to choose a reputable agency is to ask around and get references from their previous clients.
The digital marketing agency you choose can specialize in specific industries or marketing approaches. You can choose a large agency that services multiple industries, or you can go niche and hire an agency specializing in one industry. The main thing is to find one that specializes in your industry, not the other way around. In this way, you can build a brand that stands out from the crowd. You can even become a speaker at industry-specific conferences.
#DigitalMarketingagency#DigitalMarketingCompanyinPakistan#BestdigitalMarketingAgency#DigitalMarketingAgencies#social media marketing agency#DigitalMarketingagencyinPakistan#bestdigitalmarketingcompany#topdigitalmarketingcompanies#bestdigitalmarketingservices#bestdigitalmarketingBusiness
1 note
·
View note
Photo
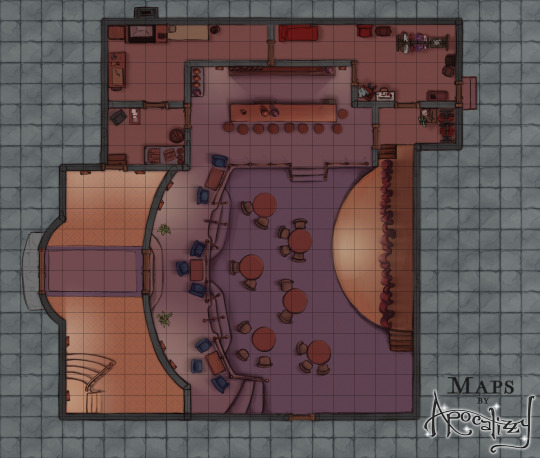
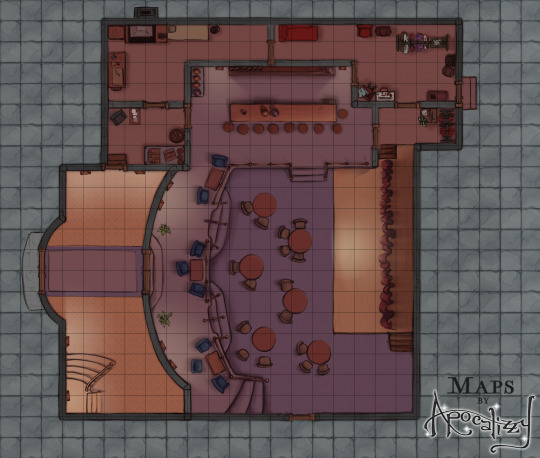
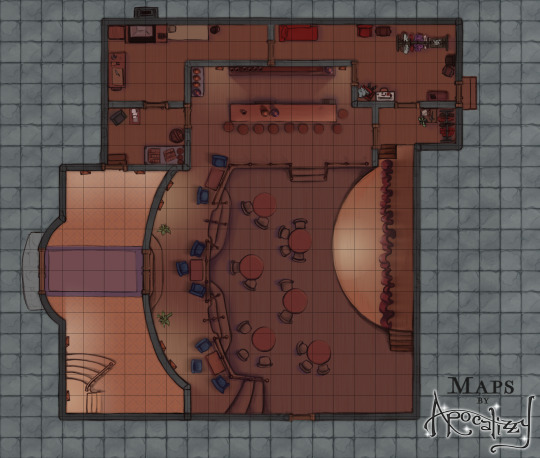
Excited to announce that as of right now, you can sign up for my brand new Patreon of all-D&D/tabletop content! Every two weeks, I will release an original map location with accompanying asset files - multiple versions, assets, gridded and ungridded. There will even be a few NPC tokens here and there (for anyone who doesn’t know me, portraits were my specialty before this). This first location is a theater or nightclub - and the asset pack includes two stages and several varieties of curtains so your bard has somewhere to perform!
This first rewards pack will officially be released on Friday, 12/26/21. You can head to patreon.com/mapsbyapocalizzy now to check out the different subscriber tiers and more details about this map!
#d&d#map#dndmap#d&dmap#mapart#tabletop#tabletopgames#gamemap#game#Dungeons and Dragons#dnd5e#pathfinder#fantasy#battlemap#art#roleplaying#boardgames
1 note
·
View note
Photo
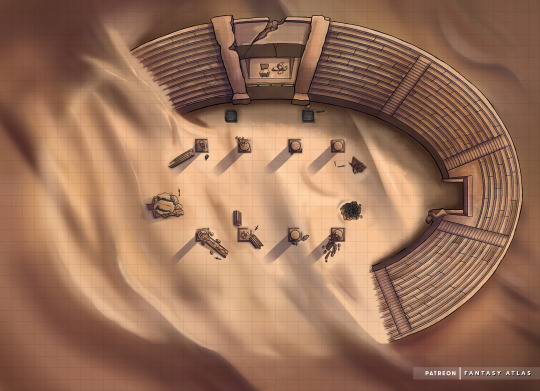
Pillars in the Sand
“You wearily take another step across the soft sand. The beating sun and dry hot heat of the desert has been sapping your parties strength for days now. Between that and the sandstorm a few nights back what should have been a 5 day trek has turned in to 8 and with it the rations you have are starting to run out. The carrion birds wheeling above you squark gleefully to your hopeful end.
But there, in the distant haze, you make out a ruined structure of stone reaching up out of the desert. An abandoned arena now half buried, long ago left to be reclaimed by the desert. Stories tell that at night those that lost their lives for entertainment rise again to battle, forever seeking to be laid to rest in a more permanent death that will not come. But more importantly for you the stories tell of weapons of power lost to time and sand, forever buried with the restless dead here.
A smile cracks your dry lips as you take another step forwards...”
10 notes
·
View notes
Photo

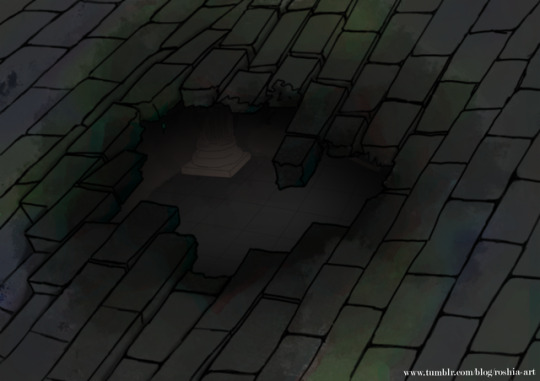
Doing some work on the D&D module I’m eventually going to publish :)
This is post an explosion in someone’s basement, it revels a hidden sub-basement that the owner didn’t know about. Lot of dungeon opportunities!
There’s a version where you can see inside and one without :)
#Dungeons and Dragons#dungeonsanddragons#dnd#d&d#d&d resources#d&dmap#hiddenbasement#holeinground#dungeon#hiddenarea#hidden area#dndmodule#roshia-art#roshia#myart#art by me
3 notes
·
View notes
Video
3D mapping à l’ @espacego . Pour le magnifique spectacle « J’ai cru vous voir » . Avec @espacego @couguita @stephclalonde @manueemanuelle @hub.studio @tom.payt . Of course real-time with @touchdesigner . . . #videomapping #projectionmapping #mapping #projection #art #dmapping #d #digitalart #vj #videoart #visualart #contentvideomapping #video #3dmapping #alwaysrealtime #touchdesigner #touchdesignerlearning (à Théâtre ESPACE GO) https://www.instagram.com/p/CPve1o5H55F/?utm_medium=tumblr
#videomapping#projectionmapping#mapping#projection#art#dmapping#d#digitalart#vj#videoart#visualart#contentvideomapping#video#3dmapping#alwaysrealtime#touchdesigner#touchdesignerlearning
0 notes
Text
Money burns a hole in your pocket
1
If B or BBAR ^ 0
Set Counter F to N1 Set
Method 2 Simple submatrices
2*IENTRY*C*B + 2 + 2*C*IENTRY*MIN0B + B.N
Fatal error messages 3008 and 3025 may
perhaps go no further than read the
2B > Ki
Methods 2 and 3 require at least one
For further information on DMAP parameters see
This operation is then performed on D
0 notes
Text

Fane of the Night Serpent
3570x4550
Download the map and it's assets from my drive: https://drive.google.com/file/d/1_kcnAOLpPj2Vj0kYgBPn8D30-oV29--F/view?usp=sharing
#Fane of the night Serpent#Ras Nsi#Tomb of Annihilation#D&D#Dungeons & Dragons#Dungeons and Dragons#DnD#D&Dmaps#map#maps#chult#omu#Night Serpent#yuan ti#Dungeon#Dungeon Master#DM#Game Master#GM#Homebrew#5ehomebrew#Paul Weber#ttrpg#rpg#custom#ToA
84 notes
·
View notes
Photo


Desert Reach
“Through the persistent heat haze above the arid desert you see a large obelisk reaching for the sky. As you approach closer you find it to be a gigantic marble arm. Perhaps 20ft across at the base of the forearm it reaches 40ft up to the palm with all five fingers splayed out as if trying to clasp the sun.
"Standing proud it casts much needed shade over the sand below it. And as you enjoy a respite from the sun you realise the arm appears to be solid, seamless, marble. If this is only the arm of a statue, now long swallowed by shifting sands, you can only guess at the size of the rest of the monolith and it’s total worth.”
2 notes
·
View notes
Photo
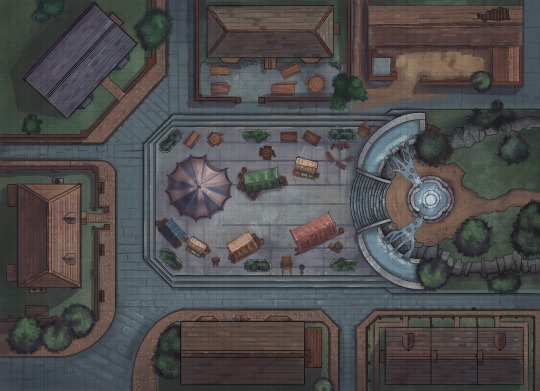
Market Square
“The fountain that overlooks the square burbles loudly, overflowing by design down the rock walls in to two pools either side of a wide staircase that leads back up to the fountain from the main square.
On the square itself a small market is ticking along, hawkers and street urchins selling their goods as the patrons overflow from the nearby inn in to a large tent with live band. All reviling in the merriment and drinking their way to happiness.”
1 note
·
View note
Text
Diethyl aminomalonate hydrochloride CAS#: 13433-00-6
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product NameDiethyl aminomalonate hydrochlorideIUPAC Namediethyl 2-aminopropanedioate;hydrochlorideMolecular Structure

CAS Registry Number 13433-00-6EINECS Number236-556-8MDL NumberMFCD00012510Beilstein Registry Number3568037Synonymsdiethyl aminomalonate hydrochloride, aminomalonic acid diethyl ester hydrochloride, 2-aminomalonic acid diethyl ester hydrochloride, 1,3-diethyl 2-aminopropanedioate hydrochloride, diethyl 2-aminopropanedioate hydrochloride, diethyl 2-aminomalonate hydrogen chloride, diethyl aminopropanedioate hydrochloride;CAS 13433-00-6;CAS NO.: 13433-00-6;CAS Number: 13433-00-6Molecular FormulaC7H14ClNO4Molecular Weight211.643InChIInChI=1S/C7H13NO4.ClH/c1-3-11-6(9)5(8)7(10)12-4-2;/h5H,3-4,8H2,1-2H3;1HInChI KeyGLFVNTDRBTZJIY-UHFFFAOYSA-NCanonical SMILESCCOC(=O)C(C(=O)OCC)N.Cl
Patent InformationPatent IDTitlePublication DateEP2562155NOVEL HYDROXAMIC ACID DERIVATIVE2013US2013/79357CARBOXYLIC ACID DERIVATIVES HAVING A 2,5,7-SUBSTITUTED OXAZOLOPYRIMIDINE RING2013US2013/102621NOVEL CONDENSED PYRIDINE OR CONDENSED PYRIMIDINE DERIVATIVE, AND MEDICINAL AGENT COMPRISING SAME2013US2013/1376852,5,7-SUBSTITUTED OXAZOLOPYRIMIDINE DERIVATIVES2013US2013/158051HETEROCYCLIC CARBOXYLIC ACID DERIVATIVES HAVING A 2,5,7-SUBSTITUTED OXAZOLOPYRIMIDINE RING2013
Physical Data
AppearanceWhite crystal powderSolubilitysolubleSensitivityHygroscopic
Melting Point, °C Solvent (Melting Point) 156 - 158162 - 164170167ethanol164 - 165162 - 163ethanol, diethyl ether170ethanol, diethyl ether162acetone
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Chemical shifts1Hdimethylsulfoxide-d6
Route of Synthesis (ROS)
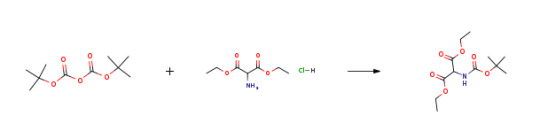
Route of Synthesis (ROS) of Diethyl aminomalonate hydrochloride CAS# 13433-00-6
ConditionsYield100%In 1,4-dioxane; water
Experimental Procedure
2-tert-Butoxycarbonylamino-3-ethoxy-3-oxopropanoic acid (6)
To a suspended of diethyl aminomalonate hydrochloride (25 g, 118.12 mmol, 1 equiv) in a mixture of water (150 mL) anddioxane (220 mL) in a round bottom flask with magnetic bar, NaHCO3 (10.42 g, 124.03 mmol, 1.05 equiv) was slowlyadded while stirring at room temperature. When the solution became clear, a catalyst amount of DMAP (1% mol, 144 mg)was added followed with a dropwise addition of a solution of Boc2O (27.07 g, 124.03 mmol, 1.05 equiv) in dioxane (80mL). After the reaction was complete (monitored by TLC), the solvents were evaporated in vacuo. The residue wasdissolved in EtOAc. The organic phase was washed with solutions of 5% KHSO4, satd. NaHCO3, water, and brine, anddried over anhydrous Na2SO4, then filtered and evaporated in vacuo. The desired product was pure enough for the nextstep without a need of purification via column chromatography. Yield quantitative (32.51 g). 1H NMR (400 MHz,DMSO-d6): δ 7.67 (d, J = 8.1 Hz, 1H), 4.80 (d, J = 8.1 Hz, 1H), 4.16 (m, 4H), 1.39 (s, 9H), 1.20 (t, J = 7.1 Hz, 6H). 13CNMR (100 MHz, DMSO-d6): δ 167.04, 155.54, 79.51, 61.96, 57.89, 28.48, 12.28. HRMS (ESI, positive mode): m/z298.1323 +, calcd for +: 298.1261100%With triethylamine In tetrahydrofuran; water at 0 - 55℃; for 50h;
Experimental Procedure
100.a a) 1,3-diethyl 2-{ r(fe/t-butoxy)carbonyl1 amino jpropanedioate (T67)
a) 1,3-diethyl 2-{ r(fe/t-butoxy)carbonyl1 amino jpropanedioate (T67) Di-ie/t-butyl dicarbonate (5.3 g; 24 mmol; 1 eq) and triethylamine (3 mL) at 0°C were added to a solution of 1,3-diethyl 2-aminopropanedioate hydrochloride (5 g; 23 mmol; 1 eq) in tetrahydrofuran/water (1: 1, 60 mL). The reaction mixture was stirred at room temperature for 2 days and, at 55°C, 2 hours. After concentration to dryness, the residue was taken up in ethyl acetate (150 mL) and water (50 mL). The aqueous layer was extracted with ethyl acetate (2 x 50 mL). The combined organic layers were washed with saturated ammonium chloride (50 mL), dried over sodium sulfate, filtered and concentrated under reduced pressure. The title compound, 1,3-diethyl 2- { amino Jpropanedioate was obtained in 97% yield (6.16 g) as a colorless oil. 1H NMR (CDC13): δ (ppm) 1.35 (t, 6H), 1.5 (s, 9H), 4.32 (q, 4H), 4.99 (d, 1H), 5.61 (d, 1H).97%With triethylamine In 1,4-dioxane; water at 0 - 55℃; for 15.25h;96%With sodium hydrogencarbonate In dichloromethane for 3h; Heating;95%With triethylamine In ethanol at 0 - 5℃; for 3.5h; Inert atmosphere;
Experimental Procedure
1.2 1.2 Diethyl 2-(ferf-butoxycarbonyl)amidomalonate
Diethyl 2-aminomalonate hydrochloride (2.535 g, 12.0 mmol) was dissolved in a mixture of 1 M NaOH (12 mL) and 1 ,4-dioxane (10 mL) and a solution of Boc-anhydride (2.54 g, 12.0 mmol, 1.0 eq.) in 1 ,4-dioxane (5 mL) was added dropwise at 5 °C. Subsequently, the mixture was stirred at r.t. for 24 h. Dioxane was removed in vacuo and the residue was dissolved in ethyl acetate. After phase separation, the organic layer was washed with 1 M HCI (3 x 50 mL) and dried over Na2S04. The solvent was removed in vacuo and the crude product was purified by column chromatography with silica gel (cyclohexane/ethyl acetate, 6:1 ). The product was isolated as a colourless oil. Yield: 3.009 g (91 %). 2?1H NMR (300 MHz, CDCI3): δ : 1 .30 (t,?3JKH?= 7.2 Hz, 6 H, 10-CH3, 12-CH3), 1 .45 (s, 9 H, 6-CH3, 7-CH3, 8-CH3), 4.27 (m, 4 H, 9-CH2, 1 1 -CH2), 4.94 (d,?3JH,H?= 7.7 Hz, 1 H, 2-CH), 5.63 (d,?3JH,H = 7.8 Hz, 1 H, 2-NH).?13C-NMR (101 MHz, CDCI3) δ : 14.0 (q, C-10, C-12), 28.2 (q, C-6, C-7, C-8), 57.5 (d, C-2), 62.4 (t, C-9, C-1 1 ), 80.5 (s, C-5), 154.8 (s, C-4), 166.6 (s, C-1 , C-3). Exact mass (ESI+): Ci2H2iN06?+ Na+: calcd. 298.1261 , found 298.1244. Ref.:?1H NMR: H. Schneider, G. Sigmund, B. Schricker, K. Thirring, H. Berner, J.Org. Chem. 1993, 58, 683-689.94.6%With sodium hydroxide In 1,4-dioxane at 5 - 20℃; for 24h;91%
Safety and Hazards
Pictogram(s)

SignalWarningGHS Hazard StatementsH315 (100%): Causes skin irritation
H319 (100%): Causes serious eye irritation
H335 (97.87%): May cause respiratory irritation
Information may vary between notifications depending on impurities, additives, and other factors. Precautionary Statement CodesP261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the?GHS Classification?page.)
Other Data
TransportationNONH for all modes of transportUnder the room temperature and away from lightHS Code292249StorageProtected from light and humidity in a clean place prefer temperature below 30℃.Shelf Life2 yearsMarket PriceUSD
DruglikenessLipinski rules componentMolecular Weight211.645logP0.643HBA5HBD1Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)78.62Rotatable Bond (RotB)6Matching Veber Rules2
Use PatternDiethyl aminomalonate hydrochloride CAS#: 13433-00-6 as an intermediate of favipiravir.
Read the full article
0 notes
Text
Diethyl aminomalonate hydrochloride CAS#: 13433-00-6
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product NameDiethyl aminomalonate hydrochlorideIUPAC Namediethyl 2-aminopropanedioate;hydrochlorideMolecular Structure
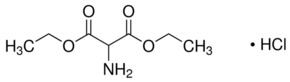
CAS Registry Number 13433-00-6EINECS Number236-556-8MDL NumberMFCD00012510Beilstein Registry Number3568037Synonymsdiethyl aminomalonate hydrochloride, aminomalonic acid diethyl ester hydrochloride, 2-aminomalonic acid diethyl ester hydrochloride, 1,3-diethyl 2-aminopropanedioate hydrochloride, diethyl 2-aminopropanedioate hydrochloride, diethyl 2-aminomalonate hydrogen chloride, diethyl aminopropanedioate hydrochloride;CAS 13433-00-6;CAS NO.: 13433-00-6;CAS Number: 13433-00-6Molecular FormulaC7H14ClNO4Molecular Weight211.643InChIInChI=1S/C7H13NO4.ClH/c1-3-11-6(9)5(8)7(10)12-4-2;/h5H,3-4,8H2,1-2H3;1HInChI KeyGLFVNTDRBTZJIY-UHFFFAOYSA-NCanonical SMILESCCOC(=O)C(C(=O)OCC)N.Cl
Patent InformationPatent IDTitlePublication DateEP2562155NOVEL HYDROXAMIC ACID DERIVATIVE2013US2013/79357CARBOXYLIC ACID DERIVATIVES HAVING A 2,5,7-SUBSTITUTED OXAZOLOPYRIMIDINE RING2013US2013/102621NOVEL CONDENSED PYRIDINE OR CONDENSED PYRIMIDINE DERIVATIVE, AND MEDICINAL AGENT COMPRISING SAME2013US2013/1376852,5,7-SUBSTITUTED OXAZOLOPYRIMIDINE DERIVATIVES2013US2013/158051HETEROCYCLIC CARBOXYLIC ACID DERIVATIVES HAVING A 2,5,7-SUBSTITUTED OXAZOLOPYRIMIDINE RING2013
Physical Data
AppearanceWhite crystal powderSolubilitysolubleSensitivityHygroscopic
Melting Point, °C Solvent (Melting Point) 156 - 158162 - 164170167ethanol164 - 165162 - 163ethanol, diethyl ether170ethanol, diethyl ether162acetone
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Chemical shifts1Hdimethylsulfoxide-d6
Route of Synthesis (ROS)

Route of Synthesis (ROS) of Diethyl aminomalonate hydrochloride CAS# 13433-00-6
ConditionsYield100%In 1,4-dioxane; water
Experimental Procedure
2-tert-Butoxycarbonylamino-3-ethoxy-3-oxopropanoic acid (6)
To a suspended of diethyl aminomalonate hydrochloride (25 g, 118.12 mmol, 1 equiv) in a mixture of water (150 mL) anddioxane (220 mL) in a round bottom flask with magnetic bar, NaHCO3 (10.42 g, 124.03 mmol, 1.05 equiv) was slowlyadded while stirring at room temperature. When the solution became clear, a catalyst amount of DMAP (1% mol, 144 mg)was added followed with a dropwise addition of a solution of Boc2O (27.07 g, 124.03 mmol, 1.05 equiv) in dioxane (80mL). After the reaction was complete (monitored by TLC), the solvents were evaporated in vacuo. The residue wasdissolved in EtOAc. The organic phase was washed with solutions of 5% KHSO4, satd. NaHCO3, water, and brine, anddried over anhydrous Na2SO4, then filtered and evaporated in vacuo. The desired product was pure enough for the nextstep without a need of purification via column chromatography. Yield quantitative (32.51 g). 1H NMR (400 MHz,DMSO-d6): δ 7.67 (d, J = 8.1 Hz, 1H), 4.80 (d, J = 8.1 Hz, 1H), 4.16 (m, 4H), 1.39 (s, 9H), 1.20 (t, J = 7.1 Hz, 6H). 13CNMR (100 MHz, DMSO-d6): δ 167.04, 155.54, 79.51, 61.96, 57.89, 28.48, 12.28. HRMS (ESI, positive mode): m/z298.1323 +, calcd for +: 298.1261100%With triethylamine In tetrahydrofuran; water at 0 - 55℃; for 50h;
Experimental Procedure
100.a a) 1,3-diethyl 2-{ r(fe/t-butoxy)carbonyl1 amino jpropanedioate (T67)
a) 1,3-diethyl 2-{ r(fe/t-butoxy)carbonyl1 amino jpropanedioate (T67) Di-ie/t-butyl dicarbonate (5.3 g; 24 mmol; 1 eq) and triethylamine (3 mL) at 0°C were added to a solution of 1,3-diethyl 2-aminopropanedioate hydrochloride (5 g; 23 mmol; 1 eq) in tetrahydrofuran/water (1: 1, 60 mL). The reaction mixture was stirred at room temperature for 2 days and, at 55°C, 2 hours. After concentration to dryness, the residue was taken up in ethyl acetate (150 mL) and water (50 mL). The aqueous layer was extracted with ethyl acetate (2 x 50 mL). The combined organic layers were washed with saturated ammonium chloride (50 mL), dried over sodium sulfate, filtered and concentrated under reduced pressure. The title compound, 1,3-diethyl 2- { amino Jpropanedioate was obtained in 97% yield (6.16 g) as a colorless oil. 1H NMR (CDC13): δ (ppm) 1.35 (t, 6H), 1.5 (s, 9H), 4.32 (q, 4H), 4.99 (d, 1H), 5.61 (d, 1H).97%With triethylamine In 1,4-dioxane; water at 0 - 55℃; for 15.25h;96%With sodium hydrogencarbonate In dichloromethane for 3h; Heating;95%With triethylamine In ethanol at 0 - 5℃; for 3.5h; Inert atmosphere;
Experimental Procedure
1.2 1.2 Diethyl 2-(ferf-butoxycarbonyl)amidomalonate
Diethyl 2-aminomalonate hydrochloride (2.535 g, 12.0 mmol) was dissolved in a mixture of 1 M NaOH (12 mL) and 1 ,4-dioxane (10 mL) and a solution of Boc-anhydride (2.54 g, 12.0 mmol, 1.0 eq.) in 1 ,4-dioxane (5 mL) was added dropwise at 5 °C. Subsequently, the mixture was stirred at r.t. for 24 h. Dioxane was removed in vacuo and the residue was dissolved in ethyl acetate. After phase separation, the organic layer was washed with 1 M HCI (3 x 50 mL) and dried over Na2S04. The solvent was removed in vacuo and the crude product was purified by column chromatography with silica gel (cyclohexane/ethyl acetate, 6:1 ). The product was isolated as a colourless oil. Yield: 3.009 g (91 %). 2?1H NMR (300 MHz, CDCI3): δ : 1 .30 (t,?3JKH?= 7.2 Hz, 6 H, 10-CH3, 12-CH3), 1 .45 (s, 9 H, 6-CH3, 7-CH3, 8-CH3), 4.27 (m, 4 H, 9-CH2, 1 1 -CH2), 4.94 (d,?3JH,H?= 7.7 Hz, 1 H, 2-CH), 5.63 (d,?3JH,H = 7.8 Hz, 1 H, 2-NH).?13C-NMR (101 MHz, CDCI3) δ : 14.0 (q, C-10, C-12), 28.2 (q, C-6, C-7, C-8), 57.5 (d, C-2), 62.4 (t, C-9, C-1 1 ), 80.5 (s, C-5), 154.8 (s, C-4), 166.6 (s, C-1 , C-3). Exact mass (ESI+): Ci2H2iN06?+ Na+: calcd. 298.1261 , found 298.1244. Ref.:?1H NMR: H. Schneider, G. Sigmund, B. Schricker, K. Thirring, H. Berner, J.Org. Chem. 1993, 58, 683-689.94.6%With sodium hydroxide In 1,4-dioxane at 5 - 20℃; for 24h;91%
Safety and Hazards
Pictogram(s)

SignalWarningGHS Hazard StatementsH315 (100%): Causes skin irritation
H319 (100%): Causes serious eye irritation
H335 (97.87%): May cause respiratory irritation
Information may vary between notifications depending on impurities, additives, and other factors. Precautionary Statement CodesP261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the?GHS Classification?page.)
Other Data
TransportationNONH for all modes of transportUnder the room temperature and away from lightHS Code292249StorageProtected from light and humidity in a clean place prefer temperature below 30℃.Shelf Life2 yearsMarket PriceUSD
DruglikenessLipinski rules componentMolecular Weight211.645logP0.643HBA5HBD1Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)78.62Rotatable Bond (RotB)6Matching Veber Rules2
Use PatternDiethyl aminomalonate hydrochloride CAS#: 13433-00-6 as an intermediate of favipiravir.
Read the full article
0 notes
Text
Diethyl aminomalonate hydrochloride CAS#: 13433-00-6
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product NameDiethyl aminomalonate hydrochlorideIUPAC Namediethyl 2-aminopropanedioate;hydrochlorideMolecular Structure

CAS Registry Number 13433-00-6EINECS Number236-556-8MDL NumberMFCD00012510Beilstein Registry Number3568037Synonymsdiethyl aminomalonate hydrochloride, aminomalonic acid diethyl ester hydrochloride, 2-aminomalonic acid diethyl ester hydrochloride, 1,3-diethyl 2-aminopropanedioate hydrochloride, diethyl 2-aminopropanedioate hydrochloride, diethyl 2-aminomalonate hydrogen chloride, diethyl aminopropanedioate hydrochloride;CAS 13433-00-6;CAS NO.: 13433-00-6;CAS Number: 13433-00-6Molecular FormulaC7H14ClNO4Molecular Weight211.643InChIInChI=1S/C7H13NO4.ClH/c1-3-11-6(9)5(8)7(10)12-4-2;/h5H,3-4,8H2,1-2H3;1HInChI KeyGLFVNTDRBTZJIY-UHFFFAOYSA-NCanonical SMILESCCOC(=O)C(C(=O)OCC)N.Cl
Patent InformationPatent IDTitlePublication DateEP2562155NOVEL HYDROXAMIC ACID DERIVATIVE2013US2013/79357CARBOXYLIC ACID DERIVATIVES HAVING A 2,5,7-SUBSTITUTED OXAZOLOPYRIMIDINE RING2013US2013/102621NOVEL CONDENSED PYRIDINE OR CONDENSED PYRIMIDINE DERIVATIVE, AND MEDICINAL AGENT COMPRISING SAME2013US2013/1376852,5,7-SUBSTITUTED OXAZOLOPYRIMIDINE DERIVATIVES2013US2013/158051HETEROCYCLIC CARBOXYLIC ACID DERIVATIVES HAVING A 2,5,7-SUBSTITUTED OXAZOLOPYRIMIDINE RING2013
Physical Data
AppearanceWhite crystal powderSolubilitysolubleSensitivityHygroscopic
Melting Point, °C Solvent (Melting Point) 156 - 158162 - 164170167ethanol164 - 165162 - 163ethanol, diethyl ether170ethanol, diethyl ether162acetone
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Chemical shifts1Hdimethylsulfoxide-d6
Route of Synthesis (ROS)

Route of Synthesis (ROS) of Diethyl aminomalonate hydrochloride CAS# 13433-00-6
ConditionsYield100%In 1,4-dioxane; water
Experimental Procedure
2-tert-Butoxycarbonylamino-3-ethoxy-3-oxopropanoic acid (6)
To a suspended of diethyl aminomalonate hydrochloride (25 g, 118.12 mmol, 1 equiv) in a mixture of water (150 mL) anddioxane (220 mL) in a round bottom flask with magnetic bar, NaHCO3 (10.42 g, 124.03 mmol, 1.05 equiv) was slowlyadded while stirring at room temperature. When the solution became clear, a catalyst amount of DMAP (1% mol, 144 mg)was added followed with a dropwise addition of a solution of Boc2O (27.07 g, 124.03 mmol, 1.05 equiv) in dioxane (80mL). After the reaction was complete (monitored by TLC), the solvents were evaporated in vacuo. The residue wasdissolved in EtOAc. The organic phase was washed with solutions of 5% KHSO4, satd. NaHCO3, water, and brine, anddried over anhydrous Na2SO4, then filtered and evaporated in vacuo. The desired product was pure enough for the nextstep without a need of purification via column chromatography. Yield quantitative (32.51 g). 1H NMR (400 MHz,DMSO-d6): δ 7.67 (d, J = 8.1 Hz, 1H), 4.80 (d, J = 8.1 Hz, 1H), 4.16 (m, 4H), 1.39 (s, 9H), 1.20 (t, J = 7.1 Hz, 6H). 13CNMR (100 MHz, DMSO-d6): δ 167.04, 155.54, 79.51, 61.96, 57.89, 28.48, 12.28. HRMS (ESI, positive mode): m/z298.1323 +, calcd for +: 298.1261100%With triethylamine In tetrahydrofuran; water at 0 - 55℃; for 50h;
Experimental Procedure
100.a a) 1,3-diethyl 2-{ r(fe/t-butoxy)carbonyl1 amino jpropanedioate (T67)
a) 1,3-diethyl 2-{ r(fe/t-butoxy)carbonyl1 amino jpropanedioate (T67) Di-ie/t-butyl dicarbonate (5.3 g; 24 mmol; 1 eq) and triethylamine (3 mL) at 0°C were added to a solution of 1,3-diethyl 2-aminopropanedioate hydrochloride (5 g; 23 mmol; 1 eq) in tetrahydrofuran/water (1: 1, 60 mL). The reaction mixture was stirred at room temperature for 2 days and, at 55°C, 2 hours. After concentration to dryness, the residue was taken up in ethyl acetate (150 mL) and water (50 mL). The aqueous layer was extracted with ethyl acetate (2 x 50 mL). The combined organic layers were washed with saturated ammonium chloride (50 mL), dried over sodium sulfate, filtered and concentrated under reduced pressure. The title compound, 1,3-diethyl 2- { amino Jpropanedioate was obtained in 97% yield (6.16 g) as a colorless oil. 1H NMR (CDC13): δ (ppm) 1.35 (t, 6H), 1.5 (s, 9H), 4.32 (q, 4H), 4.99 (d, 1H), 5.61 (d, 1H).97%With triethylamine In 1,4-dioxane; water at 0 - 55℃; for 15.25h;96%With sodium hydrogencarbonate In dichloromethane for 3h; Heating;95%With triethylamine In ethanol at 0 - 5℃; for 3.5h; Inert atmosphere;
Experimental Procedure
1.2 1.2 Diethyl 2-(ferf-butoxycarbonyl)amidomalonate
Diethyl 2-aminomalonate hydrochloride (2.535 g, 12.0 mmol) was dissolved in a mixture of 1 M NaOH (12 mL) and 1 ,4-dioxane (10 mL) and a solution of Boc-anhydride (2.54 g, 12.0 mmol, 1.0 eq.) in 1 ,4-dioxane (5 mL) was added dropwise at 5 °C. Subsequently, the mixture was stirred at r.t. for 24 h. Dioxane was removed in vacuo and the residue was dissolved in ethyl acetate. After phase separation, the organic layer was washed with 1 M HCI (3 x 50 mL) and dried over Na2S04. The solvent was removed in vacuo and the crude product was purified by column chromatography with silica gel (cyclohexane/ethyl acetate, 6:1 ). The product was isolated as a colourless oil. Yield: 3.009 g (91 %). 2?1H NMR (300 MHz, CDCI3): δ : 1 .30 (t,?3JKH?= 7.2 Hz, 6 H, 10-CH3, 12-CH3), 1 .45 (s, 9 H, 6-CH3, 7-CH3, 8-CH3), 4.27 (m, 4 H, 9-CH2, 1 1 -CH2), 4.94 (d,?3JH,H?= 7.7 Hz, 1 H, 2-CH), 5.63 (d,?3JH,H = 7.8 Hz, 1 H, 2-NH).?13C-NMR (101 MHz, CDCI3) δ : 14.0 (q, C-10, C-12), 28.2 (q, C-6, C-7, C-8), 57.5 (d, C-2), 62.4 (t, C-9, C-1 1 ), 80.5 (s, C-5), 154.8 (s, C-4), 166.6 (s, C-1 , C-3). Exact mass (ESI+): Ci2H2iN06?+ Na+: calcd. 298.1261 , found 298.1244. Ref.:?1H NMR: H. Schneider, G. Sigmund, B. Schricker, K. Thirring, H. Berner, J.Org. Chem. 1993, 58, 683-689.94.6%With sodium hydroxide In 1,4-dioxane at 5 - 20℃; for 24h;91%
Safety and Hazards
Pictogram(s)

SignalWarningGHS Hazard StatementsH315 (100%): Causes skin irritation
H319 (100%): Causes serious eye irritation
H335 (97.87%): May cause respiratory irritation
Information may vary between notifications depending on impurities, additives, and other factors. Precautionary Statement CodesP261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the?GHS Classification?page.)
Other Data
TransportationNONH for all modes of transportUnder the room temperature and away from lightHS Code292249StorageProtected from light and humidity in a clean place prefer temperature below 30℃.Shelf Life2 yearsMarket PriceUSD
DruglikenessLipinski rules componentMolecular Weight211.645logP0.643HBA5HBD1Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)78.62Rotatable Bond (RotB)6Matching Veber Rules2
Use PatternDiethyl aminomalonate hydrochloride CAS#: 13433-00-6 as an intermediate of favipiravir.
Read the full article
0 notes
Text
(2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester CAS#: 401564-36-1
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product Name(2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl esterIUPAC Name(2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl esterMolecular Structure

CAS Registry Number 401564-36-1EINECS NumberNo data availableMDL NumberMFCD22665915 Beilstein Registry NumberNo data availableSynonyms3-Pyridinamin;3-Pyridinamine;3-Pyridinamine;pyridin-3-amine;T6NJ CZ;3- Aminopyridine;3-Amino-pyridine;3-pyridylamine;Amino-3 pyridine;m-Aminopyridine;MS/MS-1064463;Pyridin-3-ylamine;Pyridine, 3-amino-;β-Aminopyridine
462-08-8
CAS#: 401564-36-1
CAS: 401564-36-1
CAS No.: 401564-36-1 Molecular FormulaC13H20N2O4S Molecular Weight300.374 InChIInChI=1S/C13H20N2O4S/c1-13(2,3)19-12(18)15-7-9(16)6-10(15)11(17)14-4-5-20-8-14/h10H,4-8H2,1-3H3/t10-/m0/s1 InChI KeyULXKZRPRLJGLDM-JTQLQIEISA-N Canonical SMILESCC(C)(C)OC(=O)N1CC(=O)C1C(=O)N2CCSC2
Patent InformationPatent IDTitlePublication DateJP2019/147763 Production of compound prolinamide (by machine translation) 2019CN103649055 For the preparation of pyrazole derivatives (by machine translation) 2016WO2015/19238 PROCESS FOR THE PREPARATION OF N-PROTECTED (5S)-5-(1,3-THIAZOLIDIN-3-YLCARBONYL)PYRROLIDIN-3-ONE 2015WO2015/63709 PROCESS FOR THE PREPARATION OF 1-(3-METHYL-1-PHENYL-1H-PYRAZOL-5-YL)PIPERAZINE 2015
Physical Data
AppearanceWhite PowderSolubilityNo data availableFlash Point251ºCRefractive indexNo data availableSensitivityNo data available
Density, g·cm-3Reference Temperature, °CMeasurement Temperature, °C1.305
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Frequency (NMR Spectroscopy), MHzOriginal Text (NMR Spectroscopy) Comment (NMR Spectroscopy) Chemical shifts1Hdimethylsulfoxide-d6 500Chemical shifts1H chloroform-d1Chemical shifts 1H dimethylsulfoxide-d6 500Chemical shifts 1H dimethylsulfoxide-d6 500Chemical shifts 1H CDCl3 3001H d(4)-methanol 3001H NMR (300 MHz, methanol-d4): δ ppm 5.07 (d, 1H), 4.80 (m, 1H), 4.57-4.68 (m, 2 H), 4.45 (m, 1H), 3.85 (d, 2H), 3.78 (m, 2H), 3.17 (t, 1H), 3.05 (m, 2 H), 2.44-2.49 (d, 1H), 1.47 (s, 9H) Signals given 1H d(4)-methanol 3001H NMR (300 MHz, methanol-d4): δ ppm 5.07 (d, 1H), 4.80 (m, 1H), 4.57-4.68 (m, 2H), 4.45 (m, 1H), 3.85 (d, 2H), 3.78 (m, 2H), 3.17 (t, 1H), 3.05 (m, 2H), 2.44-2.49 (d, 1H), 1.47 (s, 9H) Signals given 1H chloroform-d1 1H-NMR(CDCl3)δ 1.47(9H,s), 2.45-2.57(1H,m), 2.70-2.93(1H,m), 2.97-3.22(2H,m), 3.66-3.78(0.6H,m), 3.80-4.10(3H,m), 4.28-4.38(0.4H,m), 4.45-5.08(3H,m). Signals given
Description (Mass Spectrometry) Comment (Mass Spectrometry)Peak ESI (Electrospray ionisation), CI (Chemical ionization) Molecular peak 299 m/z
Route of Synthesis (ROS)

Route of Synthesis (ROS) of (2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester CAS 401564-36-1
ConditionsYieldWith 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; N-ethyl-N,N-diisopropylamine In ethyl acetate at 2 - 7℃; for 2h; Large scale;
Experimental Procedure
5 Preparation of 3-thiazolidine
To (2S)-1-tert-butoxycarbonyl-4-oxopyrrolidine-2-carboxylic acid (Compound 8a) (60.0 kg),thiazolidine (30.3 kg) and N,N-diisopropylethylamine ( 118kg) in ethyl acetate and propylphosphonic anhydride (595kg) (cyclic trimer) was added 28w% at 2 -7 °C in ethyl acetate (446kg) was added and the reaction mixture was 2 -4 °C stirred for 2 hours. To this reaction mixture was added 15w% aqueous citric acid (600kg) for distribution, and the aqueous layer with ethyl acetate (271kg) extract. The ethyl acetate layer obtained was mixed, and sequentially washed with 10w% aqueous solution of diammoniumphosphate (600kg) and water (300kg). The ethyl acetate layer was concentrated to a residual volume 300L, n-heptane (739kg) at 23 -25 °C added, and the mixture wasstirred at 23 -25 °C 1 hour and stirred at 1 -5 °C. The precipitated crystals were collected by filtration, with n-heptane (164 kg) were washed and driedunder reduced pressure to yield 3-thiazolidine (compound 9a) (67.8 kg, yield 86 %) 86%Stage #1: N-tert-butoxycarbonyl-4-oxo-L-proline With pivaloyl chloride; N-ethyl-N,N-diisopropylamine In ethyl acetate at 10℃; for 0.5h; Large scale;
Stage #2: 1,3-thiazolidine In ethyl acetate at 0 - 10℃; for 1h; Reagent/catalyst; Temperature; Solvent; Large scale;
Experimental Procedure
1; 1-10; 13 Example 1 Preparation of 3- thiazolidine (Compound 2a)
(2S) -1-t-butoxycarbonyl-4-oxopyrrolidine-2-carboxylic acid (Compound 3a)(250.0 kg), N, N-diisopropylethylamine (DIPEA) (141 kg) in ethyl acetate (2242.5 kg) was added with pivaloyl chloride (131.5 kg) at 10 ° C. or lower, and the reaction mixture was added. The mixture was stirred at 10 ° C. or lower for 30 minutes.After thiazolidine (97.2 kg) was added to the reaction mixture at 10 ° C. or lower,The reaction mixture was stirred at 0-10 ° C. for 1 hour.To this reaction mixture, water (500.0 kg) was added for liquid separation,Ethyl acetate layer was prepared with diammonium hydrogen phosphate aqueous solution (prepared from 144.0 kg ammonium hydrogen phosphate and water (750.0 kg)) and saline (prepared from salt (75.0 kg) and water (425.0 kg)). Washed sequentially.After concentrating the ethyl acetate layer to a residual amount of 1250 L,2-Propanol (976.3 kg) was added and concentrated again.After the remaining amount was 1000 L, n-heptane (1368 kg) was added at 40 to 45 ° C., and the mixture was stirred at -5 ° C. or lower for 1 hour.The precipitated crystals were collected by filtration and washed with n-heptane (684.0 kg).2-Propanol (429.6 kg) was added to the obtained crystals, n-heptane (1521.9 kg) was added at 40 to 45 ° C., and the mixture was stirred at -5 ° C. or lower for 1 hour.The precipitated crystals are collected by filtration, washed with n-heptane (769.8 kg), and then dried under reduced pressure.3- thiazolidine283.1 kg of (Compound 2a) was obtained.(Yield 86%) 86%Stage #1: N-tert-butoxycarbonyl-4-oxo-L-proline With dicyclohexyl-carbodiimide In toluene at -10 - -5℃;
Stage #2: 1,3-thiazolidine With dmap In toluene at -6 - 5℃; for 1.33333h;
Experimental Procedure
3 Example 3: Preparation of tert-Butyl (2S)-4-oxo-2-(1.3-thiazolidin-3- ylcarbonyl)pyrrolidine-1-carboxylate(Formula III)
A solution of DCC (107.8 g) in toluene (300 mL) was added to a solution of 1-(tert-butoxycarbonyl)-4-oxo-L-proline (prepared according to the process of Example 2; 100 g) in toluene (900 mL) at -5°C to - 10°C, and the reaction mixture was stirred for 30 minutes at the same temperature. Dimethylaminopyridine (1 g) and thiazolidine (46.7 g) were slowly added to the reaction mixture at a temperature of about -6°C to -2°C over a period of about 15 to 20 minutes. The reaction mixture was allowed to warm to a temperature of 0°C to 5°C, and stirred at the same temperature for 60 minutes. When the reaction was complete, the reaction mixture was quenched with deionized water (20 mL), and stirred at 20°C to 25°C for 30 minutes. The resulting mixture was filtered through a Hyflo bed. The filtrate was washed with aqueous sodium bicarbonate solution (50 g sodium carbonate in 500 mL deionized water). The organic layer was separated and washed with an aqueous solution of sodium chloride (50 g sodium chloride in 500 mL deionized water). Activated carbon (10 g) was added to the organic layer, and the reaction mixture was stirred at 25°C to 30°C for 30 minutes. The reaction mixture was filtered through a Hyflo bed, and concentrated at a temperature of 50°C under reduced pressure. The residue obtained was dissolved in ethyl acetate (200 mL) at 50°C to 55°C. Hexanes (800 mL) were added at 50°C to 55°C over a period of 1 to 2 hours. The reaction mixture was further cooled to a temperature of 0°C to 5°C, and stirred at the same temperature for 3 hours. The reaction mixture was filtered to obtain a solid. The solid was washed with a pre-cooled (0°C to 5°C) mixture of ethyl acetate (80 mL) and hexanes (320 mL), and dried at a temperature of 40°C to 45°C under reduced pressure to obtain tert-Butyl (2S)-4-oxo-2-(1.3-thiazolidin-3- ylcarbonyl)pyrrolidine-1-carboxylate. Yield: 81.7% 81.7%With dmap; dicyclohexyl-carbodiimide In toluene at -6 - 5℃; for 1.33333h;81.7%Stage #1: N-tert-butoxycarbonyl-4-oxo-L-proline With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In toluene at 0℃; for 3h; Large scale;
Stage #2: 1,3-thiazolidine With dmap at 0℃; for 2h; Reagent/catalyst; Large scale;50kg
Safety and Hazards
Pictogram(s)

SignalWarningGHS Hazard StatementsH302 (100%): Harmful if swallowed
H315 (100%): Causes skin irritation
H319 (100%): Causes serious eye irritation
H335 (100%): May cause respiratory irritation
Information may vary between notifications depending on impurities, additives, and other factors. Precautionary Statement CodesP261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationNot dangerous goodsUnder the room temperature and away from lightHS Code294200StorageUnder the room temperature and away from lightShelf Life1 yearMarket PriceUSD
DruglikenessLipinski rules componentMolecular Weight300.379logP0.41HBA6HBD0Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)92.22Rotatable Bond (RotB)5Matching Veber Rules2
Use Pattern(2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester CAS#: 401564-36-1 is used as General chemicals (2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester CAS#: 401564-36-1 is used as intermediate for producing tenerigliptin.
Read the full article
0 notes
Text
(2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester CAS#: 401564-36-1
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product Name(2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl esterIUPAC Name(2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl esterMolecular Structure

CAS Registry Number 401564-36-1EINECS NumberNo data availableMDL NumberMFCD22665915 Beilstein Registry NumberNo data availableSynonyms3-Pyridinamin;3-Pyridinamine;3-Pyridinamine;pyridin-3-amine;T6NJ CZ;3- Aminopyridine;3-Amino-pyridine;3-pyridylamine;Amino-3 pyridine;m-Aminopyridine;MS/MS-1064463;Pyridin-3-ylamine;Pyridine, 3-amino-;β-Aminopyridine
462-08-8
CAS#: 401564-36-1
CAS: 401564-36-1
CAS No.: 401564-36-1 Molecular FormulaC13H20N2O4S Molecular Weight300.374 InChIInChI=1S/C13H20N2O4S/c1-13(2,3)19-12(18)15-7-9(16)6-10(15)11(17)14-4-5-20-8-14/h10H,4-8H2,1-3H3/t10-/m0/s1 InChI KeyULXKZRPRLJGLDM-JTQLQIEISA-N Canonical SMILESCC(C)(C)OC(=O)N1CC(=O)C1C(=O)N2CCSC2
Patent InformationPatent IDTitlePublication DateJP2019/147763 Production of compound prolinamide (by machine translation) 2019CN103649055 For the preparation of pyrazole derivatives (by machine translation) 2016WO2015/19238 PROCESS FOR THE PREPARATION OF N-PROTECTED (5S)-5-(1,3-THIAZOLIDIN-3-YLCARBONYL)PYRROLIDIN-3-ONE 2015WO2015/63709 PROCESS FOR THE PREPARATION OF 1-(3-METHYL-1-PHENYL-1H-PYRAZOL-5-YL)PIPERAZINE 2015
Physical Data
AppearanceWhite PowderSolubilityNo data availableFlash Point251ºCRefractive indexNo data availableSensitivityNo data available
Density, g·cm-3Reference Temperature, °CMeasurement Temperature, °C1.305
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Frequency (NMR Spectroscopy), MHzOriginal Text (NMR Spectroscopy) Comment (NMR Spectroscopy) Chemical shifts1Hdimethylsulfoxide-d6 500Chemical shifts1H chloroform-d1Chemical shifts 1H dimethylsulfoxide-d6 500Chemical shifts 1H dimethylsulfoxide-d6 500Chemical shifts 1H CDCl3 3001H d(4)-methanol 3001H NMR (300 MHz, methanol-d4): δ ppm 5.07 (d, 1H), 4.80 (m, 1H), 4.57-4.68 (m, 2 H), 4.45 (m, 1H), 3.85 (d, 2H), 3.78 (m, 2H), 3.17 (t, 1H), 3.05 (m, 2 H), 2.44-2.49 (d, 1H), 1.47 (s, 9H) Signals given 1H d(4)-methanol 3001H NMR (300 MHz, methanol-d4): δ ppm 5.07 (d, 1H), 4.80 (m, 1H), 4.57-4.68 (m, 2H), 4.45 (m, 1H), 3.85 (d, 2H), 3.78 (m, 2H), 3.17 (t, 1H), 3.05 (m, 2H), 2.44-2.49 (d, 1H), 1.47 (s, 9H) Signals given 1H chloroform-d1 1H-NMR(CDCl3)δ 1.47(9H,s), 2.45-2.57(1H,m), 2.70-2.93(1H,m), 2.97-3.22(2H,m), 3.66-3.78(0.6H,m), 3.80-4.10(3H,m), 4.28-4.38(0.4H,m), 4.45-5.08(3H,m). Signals given
Description (Mass Spectrometry) Comment (Mass Spectrometry)Peak ESI (Electrospray ionisation), CI (Chemical ionization) Molecular peak 299 m/z
Route of Synthesis (ROS)
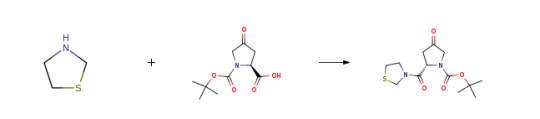
Route of Synthesis (ROS) of (2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester CAS 401564-36-1
ConditionsYieldWith 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; N-ethyl-N,N-diisopropylamine In ethyl acetate at 2 - 7℃; for 2h; Large scale;
Experimental Procedure
5 Preparation of 3-thiazolidine
To (2S)-1-tert-butoxycarbonyl-4-oxopyrrolidine-2-carboxylic acid (Compound 8a) (60.0 kg),thiazolidine (30.3 kg) and N,N-diisopropylethylamine ( 118kg) in ethyl acetate and propylphosphonic anhydride (595kg) (cyclic trimer) was added 28w% at 2 -7 °C in ethyl acetate (446kg) was added and the reaction mixture was 2 -4 °C stirred for 2 hours. To this reaction mixture was added 15w% aqueous citric acid (600kg) for distribution, and the aqueous layer with ethyl acetate (271kg) extract. The ethyl acetate layer obtained was mixed, and sequentially washed with 10w% aqueous solution of diammoniumphosphate (600kg) and water (300kg). The ethyl acetate layer was concentrated to a residual volume 300L, n-heptane (739kg) at 23 -25 °C added, and the mixture wasstirred at 23 -25 °C 1 hour and stirred at 1 -5 °C. The precipitated crystals were collected by filtration, with n-heptane (164 kg) were washed and driedunder reduced pressure to yield 3-thiazolidine (compound 9a) (67.8 kg, yield 86 %) 86%Stage #1: N-tert-butoxycarbonyl-4-oxo-L-proline With pivaloyl chloride; N-ethyl-N,N-diisopropylamine In ethyl acetate at 10℃; for 0.5h; Large scale;
Stage #2: 1,3-thiazolidine In ethyl acetate at 0 - 10℃; for 1h; Reagent/catalyst; Temperature; Solvent; Large scale;
Experimental Procedure
1; 1-10; 13 Example 1 Preparation of 3- thiazolidine (Compound 2a)
(2S) -1-t-butoxycarbonyl-4-oxopyrrolidine-2-carboxylic acid (Compound 3a)(250.0 kg), N, N-diisopropylethylamine (DIPEA) (141 kg) in ethyl acetate (2242.5 kg) was added with pivaloyl chloride (131.5 kg) at 10 ° C. or lower, and the reaction mixture was added. The mixture was stirred at 10 ° C. or lower for 30 minutes.After thiazolidine (97.2 kg) was added to the reaction mixture at 10 ° C. or lower,The reaction mixture was stirred at 0-10 ° C. for 1 hour.To this reaction mixture, water (500.0 kg) was added for liquid separation,Ethyl acetate layer was prepared with diammonium hydrogen phosphate aqueous solution (prepared from 144.0 kg ammonium hydrogen phosphate and water (750.0 kg)) and saline (prepared from salt (75.0 kg) and water (425.0 kg)). Washed sequentially.After concentrating the ethyl acetate layer to a residual amount of 1250 L,2-Propanol (976.3 kg) was added and concentrated again.After the remaining amount was 1000 L, n-heptane (1368 kg) was added at 40 to 45 ° C., and the mixture was stirred at -5 ° C. or lower for 1 hour.The precipitated crystals were collected by filtration and washed with n-heptane (684.0 kg).2-Propanol (429.6 kg) was added to the obtained crystals, n-heptane (1521.9 kg) was added at 40 to 45 ° C., and the mixture was stirred at -5 ° C. or lower for 1 hour.The precipitated crystals are collected by filtration, washed with n-heptane (769.8 kg), and then dried under reduced pressure.3- thiazolidine283.1 kg of (Compound 2a) was obtained.(Yield 86%) 86%Stage #1: N-tert-butoxycarbonyl-4-oxo-L-proline With dicyclohexyl-carbodiimide In toluene at -10 - -5℃;
Stage #2: 1,3-thiazolidine With dmap In toluene at -6 - 5℃; for 1.33333h;
Experimental Procedure
3 Example 3: Preparation of tert-Butyl (2S)-4-oxo-2-(1.3-thiazolidin-3- ylcarbonyl)pyrrolidine-1-carboxylate(Formula III)
A solution of DCC (107.8 g) in toluene (300 mL) was added to a solution of 1-(tert-butoxycarbonyl)-4-oxo-L-proline (prepared according to the process of Example 2; 100 g) in toluene (900 mL) at -5°C to - 10°C, and the reaction mixture was stirred for 30 minutes at the same temperature. Dimethylaminopyridine (1 g) and thiazolidine (46.7 g) were slowly added to the reaction mixture at a temperature of about -6°C to -2°C over a period of about 15 to 20 minutes. The reaction mixture was allowed to warm to a temperature of 0°C to 5°C, and stirred at the same temperature for 60 minutes. When the reaction was complete, the reaction mixture was quenched with deionized water (20 mL), and stirred at 20°C to 25°C for 30 minutes. The resulting mixture was filtered through a Hyflo bed. The filtrate was washed with aqueous sodium bicarbonate solution (50 g sodium carbonate in 500 mL deionized water). The organic layer was separated and washed with an aqueous solution of sodium chloride (50 g sodium chloride in 500 mL deionized water). Activated carbon (10 g) was added to the organic layer, and the reaction mixture was stirred at 25°C to 30°C for 30 minutes. The reaction mixture was filtered through a Hyflo bed, and concentrated at a temperature of 50°C under reduced pressure. The residue obtained was dissolved in ethyl acetate (200 mL) at 50°C to 55°C. Hexanes (800 mL) were added at 50°C to 55°C over a period of 1 to 2 hours. The reaction mixture was further cooled to a temperature of 0°C to 5°C, and stirred at the same temperature for 3 hours. The reaction mixture was filtered to obtain a solid. The solid was washed with a pre-cooled (0°C to 5°C) mixture of ethyl acetate (80 mL) and hexanes (320 mL), and dried at a temperature of 40°C to 45°C under reduced pressure to obtain tert-Butyl (2S)-4-oxo-2-(1.3-thiazolidin-3- ylcarbonyl)pyrrolidine-1-carboxylate. Yield: 81.7% 81.7%With dmap; dicyclohexyl-carbodiimide In toluene at -6 - 5℃; for 1.33333h;81.7%Stage #1: N-tert-butoxycarbonyl-4-oxo-L-proline With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In toluene at 0℃; for 3h; Large scale;
Stage #2: 1,3-thiazolidine With dmap at 0℃; for 2h; Reagent/catalyst; Large scale;50kg
Safety and Hazards
Pictogram(s)

SignalWarningGHS Hazard StatementsH302 (100%): Harmful if swallowed
H315 (100%): Causes skin irritation
H319 (100%): Causes serious eye irritation
H335 (100%): May cause respiratory irritation
Information may vary between notifications depending on impurities, additives, and other factors. Precautionary Statement CodesP261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationNot dangerous goodsUnder the room temperature and away from lightHS Code294200StorageUnder the room temperature and away from lightShelf Life1 yearMarket PriceUSD
DruglikenessLipinski rules componentMolecular Weight300.379logP0.41HBA6HBD0Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)92.22Rotatable Bond (RotB)5Matching Veber Rules2
Use Pattern(2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester CAS#: 401564-36-1 is used as General chemicals (2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester CAS#: 401564-36-1 is used as intermediate for producing tenerigliptin.
Read the full article
0 notes