#Micropulse Inc.
Text
Nu Skin Wins “New Product of the Year” BIG Award
Nu Skin Wins “New Product of the Year” BIG Award
Nu Skin Enterprises, Inc. was honored at the annual BIG Awards for Business for its ageLOC LumiSpa iO, earning a 2022 “New Product of the Year” award.
The BIG Awards for Business recognizes products and companies who endeavor to change the way people experience the world by bringing new ideas to life.
“The ageLOC LumiSpa iO is a one-of-a-kind beauty and cleansing device with patented micropulse…
View On WordPress
0 notes
Text
Ivy Tech community job fairs - April 2021
New Post has been published on https://aroundfortwayne.com/news/2021/04/02/ivy-tech-community-job-fairs-april-2021/
Ivy Tech community job fairs - April 2021

Ivy Tech Fort Wayne and Warsaw invite the community to two career fairs in April 2021.
#80/20 Inc.#A Caring Hand LLC#Advanced Technological Solutions#Anh Dinh Lapsley#Belle Tire#Fort Wayne Indiana#Fort Wayne/Warsaw MadeByMe Manufacturing Virtual Fair#Greater Fort Wayne Inc.#Ivy Tech Community College#Kelley Automotive Group#KGPCo#Lutheran Life Villages#Micropulse#Northern Lakes Nursing and Rehabilitation Center#Otis R. Bowen Center for Human Services#Parkview Health#Peabody Retirement Community#Preferred Inc.#Premier Truck Rental#QuikCut#Republic Services#ScribeAmerica#SDI Steel Dynamics Inc.#The Healthcare and Human Services Career Fair#Warsaw Indiana
0 notes
Text
Macular Edema Treatment Market Boosting The Growth: Market Dynamics And Trends, Efficiencies Forecast

Macular edema is the condition characterized by abnormal leakage and accumulation of fluid that forms blister in the layer of macula. The macula is an oval-shaped pigmented area near the center of retina. This blister causes the macula to swell and thicken that causes distortion of vision. Although macular edema has numerous causes, the most common cause is diabetes. Other causes include, age-related macular degeneration, replacement of lenses in treatment of cataract, chronic uveitis and intermediate uveitis, blockage of vein in the retina, and congenital diseases including retinitis pigmentosa and retinoschisis. Symptoms of macular edema include blurry or wavy vision near or in the center of field of vision. The disease is diagnosed by performing tests such as visual acuity test, dilated eye exam, fluorescein angiogram, optical coherence tomography, and Amsler grid.
Get Exclusive Sample Report at: http://bit.ly/2IgbA7y
Macular Edema Treatment Market: Drivers
Increasing number of pipeline studies is expected to drive the macular edema treatment market growth. For instance, in April 2018, University of California, Davis, in collaboration with IRIDEX Corporation, initiated phase 3 clinical trial for Micropulse Laser Treatment to evaluate micropulse for suppression of diabetic macular edema (pulse). The primary aim of the study is to determine whether the micropulse laser treatment in eyes with good visual acuity will improve or stabilize vision loss due to the complications of diabetic macular edema. The study is expected to be completed by December 2020.
Furthermore, increasing approval for new diabetic macular edema (DME) therapies is expected to propel growth for the macular edema treatment market. For instance, in March 2018, U.S. Food and Drug Administration (FDA) approved Lucentis (ranibizumab injection) 0.3 mg prefilled syringe (PFS) for diabetic macular edema and diabetic retinopathy. Lucentis 0.3 mg PFS is the first syringe prefilled with an anti-vascular endothelial growth factor (VEGF) agent. Moreover, in 2016 U.S.FDA approved Lucentis 0.5 mg PFS, which is intended for use in the treatment of neovascular (wet) age-related macular degeneration (AMD), macular edema following retinal vein occlusion (RVO), and myopic choroidal neovascularisation (mCNV).
However, lack of awareness of diabetic eye diseases among the patients with diabetes is expected to hinder the macular edema treatment market growth.
Macular Edema Treatment Market: Regional Analysis
On the basis of region, the global macular edema treatment market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa.
North America is expected to account for the largest market share in the macular edema treatment market, owing to increasing prevalence of diabetic macular edema in this region. For instance, according to American Journal of Managed Care (AJMC), in 2016, the prevalence rate of diabetic macular edema was 45.3% in North America.
Moreover, in 2013, as per National Center for Biotechnology Information (NCBI), around 98 million individuals reported for diabetic retinopathy (DR) and 21 million individual reported for diabetic macular edema (DME) worldwide.
Furthermore, increasing product approvals for the treatment of macular edema is expected to boost growth of the macular edema treatment market in Europe. For instance, in 2015, the European Commission approved Eylea (aflibercept) for the treatment of patients with macular edema secondary to retinal vein occlusion. Bayer HealthCare and Regeneron collaborated to develop Eylea. Bayer HealthCare has exclusive marketing rights to the product outside the U.S.
Ask For Discount Before Purchasing This Business Report @ http://bit.ly/31wHqo1
Key players operating in the macular edema treatment market include, Novartis International AG, F. Hoffmann-La Roche Ltd, Regeneron Pharmaceuticals, Inc., Pfizer Inc. Bayer AG, Aerpio Therapeutics, Inc., Biomar Microbial Technologies, Antisense Therapeutics Limited, Coherus BioSciences, and Bausch & Lomb Incorporated.
Macular Edema Treatment Market Taxonomy
By Disease Type
Diabetic Macular Edema
Cystoid Macular Edema
By Drug Class
Anti-Vascular endothelial growth factor (VEGF) injections
Avastin
Eylea
Lucentis
Anti-inflammatory Medication
Nonsteroidal anti-inflammatory drugs (NSAIDs)
Corticosteroid
Ozurdex
Retisert
Iluvien
By End User
Hospitals
Ophthalmic Clinics
Research Institutes
By Region
North America
Europe
Asia Pacific
Latin America
Middle East
Africa
About Coherent Market Insights
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us
Mr. Shah
Coherent Market Insights
1001 4th Ave, #3200
Seattle, WA 98154
Tel: +1-206-701-6702
Email: [email protected]
0 notes
Text
Macular Edema Treatment Market Benefit and Volume 2018 with Status and Prospect to 2026
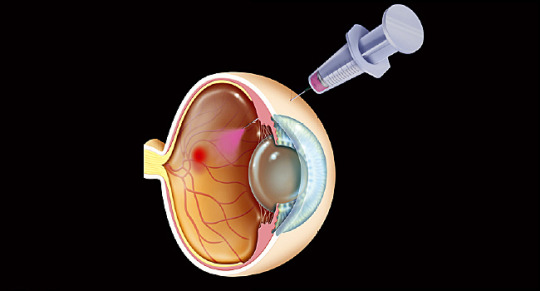
Macular edema is the condition characterized by abnormal leakage and accumulation of fluid that forms blister in the layer of macula. The macula is an oval-shaped pigmented area near the center of retina. This blister causes the macula to swell and thicken that causes distortion of vision. Although macular edema has numerous causes, the most common cause is diabetes. Other causes include, age-related macular degeneration, replacement of lenses in treatment of cataract, chronic uveitis and intermediate uveitis, blockage of vein in the retina, and congenital diseases including retinitis pigmentosa and retinoschisis. Symptoms of macular edema include blurry or wavy vision near or in the center of field of vision. The disease is diagnosed by performing tests such as visual acuity test, dilated eye exam, fluorescein angiogram,optical coherence tomography, and Amsler grid. Click To Read More On Macular Edema Treatment Market.
Macular Edema Treatment Market: Drivers
Increasing number of pipeline studies is expected to drive the macular edema treatment market growth. For instance, in April 2018, University of California, Davis, in collaboration with IRIDEX Corporation, initiated phase 3 clinical trial for Micropulse Laser Treatment to evaluate micropulse for suppression of diabetic macular edema (pulse). The primary aim of the study is to determine whether the micropulse laser treatment in eyes with good visual acuity will improve or stabilize vision loss due to the complications of diabetic macular edema. The study is expected to be completed by December 2020.
Furthermore, increasing approval for new diabetic macular edema (DME) therapies is expected to propel growth for the macular edema treatment market. For instance, in March 2018, U.S. Food and Drug Administration (FDA) approved Lucentis (ranibizumab injection) 0.3 mg prefilled syringe (PFS) for diabetic macular edema and diabetic retinopathy. Lucentis 0.3 mg PFS is the first syringe prefilled with an anti-vascular endothelial growth factor (VEGF) agent. Moreover, in 2016 U.S.FDA approved Lucentis 0.5 mg PFS, which is intended for use in the treatment of neovascular (wet) age-related macular degeneration (AMD), macular edema following retinal vein occlusion (RVO), and myopic choroidal neovascularisation (mCNV).
Macular Edema Treatment Market: Regional Analysis
On the basis of region, the global macular edema treatment market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa.
North America is expected to account for the largest market share in the macular edema treatment market, owing to increasing prevalence of diabetic macular edema in this region. For instance, according to American Journal of Managed Care (AJMC), in 2016, the prevalence rate of diabetic macular edema was 45.3% in North America.
Ask For Sample Copy Of This Business Research Report : https://www.coherentmarketinsights.com/insight/request-sample/2494
Moreover, in 2013, as per National Center for Biotechnology Information (NCBI), around 98 million individuals reported for diabetic retinopathy (DR) and 21 million individual reported for diabetic macular edema (DME) worldwide.
Furthermore, increasing product approvals for the treatment of macular edema is expected to boost growth of the macular edema treatment market in Europe. For instance, in 2015, the European Commission approved Eylea (aflibercept) for the treatment of patients with macular edema secondary to retinal vein occlusion. Bayer HealthCare and Regeneron collaborated to develop Eylea. Bayer HealthCare has exclusive marketing rights to the product outside the U.S.
Competitive Analysis
Key players operating in the macular edema treatment market include, Novartis International AG, F. Hoffmann-La Roche Ltd, Regeneron Pharmaceuticals, Inc., Pfizer Inc. Bayer AG, Aerpio Therapeutics, Inc., Biomar Microbial Technologies, Antisense Therapeutics Limited, Coherus BioSciences, and Bausch & Lomb Incorporated.
Also, Coherent Market Insights has a proprietary database of pipeline biologics and biosimilars, called PHASE-XS. This database provides analytical data in addition to the clinical information of ongoing trials for biologics and biosimilars. An amalgamation of more than 30 parameters, PHASE-XS helps biotechnology and pharmaceutical companies to analyze the market trend, competition, and market potential. For more information or to access this database, kindly click on the below link or contact at [email protected]
https://www.coherentmarketinsights.com/phase-xs/
About Coherent Market Insights:
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us:
Mr. Shah
Coherent Market Insights
1001 4th Ave,
#3200
Seattle, WA 98154
Tel: +1-206-701-6702
Email: [email protected]
#Macular Edema Treatment Market#Macular Edema Treatment Market Size#Macular Edema Treatment Market Share#Macular Edema Treatment Market Outlook
0 notes
Text
Refractory Glaucoma Treatment Market Advancements to Watch Out For 2026
Globally, Glaucoma is on rise. According to the Glaucoma research Foundation, Glaucoma is the leading cause of irreversible blindness globally. Nearly 10% of the glaucoma patients who receive proper treatment still experience a loss of vision. Around 64.3 million people were estimated to be having glaucoma globally in 2013. Furthermore, according to the American Academy of Ophthalmology, the current prevalence is high in Africa, and Latin America. Patients who fail to respond to medications, undergo trabeculectomy surgery. However, few patients do not get completely relieved from glaucoma even after trabeculectomy surgery. Therefore, novel treatment options are required to be introduced for providing relief to such patients. Reducing intra-ocular pressure is the most important long term medical need of refractory glaucoma patients.
Request For a Sample Copy of This Business Report @ https://www.coherentmarketinsights.com/insight/request-sample/71
Refractory Glaucoma Treatment Options
The global refractory glaucoma treatment market can be characterized based on the treatment options and devices available in the market.
Ahmed Glaucoma Valve from New World Medical Inc., is a good treatment option available since 1993 for refractory glaucoma. This device also has applications in primary open angle glaucoma unresponsive to medication, neovascular glaucoma, and congenital or infantile glaucoma. Various studies have reflected the positive outcomes of using Ahmed Glaucoma Valve in refractory glaucoma both in pediatrics and adults. Therefore, this device is in very high demand and usage globally.
Alternate option like CyPass Micro-Stent from Transcend Medical showed impressive results in patients with primary open-angle glaucoma. CyPass is a micro-invasive surgery which according to the FDA guidelines needs to be performed in combination with cataract surgery. This is restricting the wider acceptance among patients due to increased cost.
Clinical trials of Xen System showed positive results with 44% reduction in eye pressure and 65% reduction in IOP medications in one year period. The Xen gel stent form Allergan reduces IOP with a permanent implant that becomes flexible, allowing doctors to use IOP reduction therapies event after the Xen implantation. Allergan received U.S. FDA approval for Xen Gel stent in November 2016. The company plans to launch the Xen Gel stent in the U.S. market, for refractory glaucoma treatment, in early 2017. The Xen system has already received CE mark and has distributed more than 10,500 stents globally. Moreover, the new surgical system is also licensed for use in Canada, Switzerland, and Turkey.
Future Market Dynamics
According to the American Academy of Ophthalmology, the global prevalence of glaucoma is estimated to rise to 76 million in 2020 and 111.8 million in 2040. Rapidly aging population will be one of the important factors for the increasing prevalence rate. There would be a disproportionate rise in the prevalence in people residing in Asia and Africa. Slower adoption rate and high cost of the surgery is limiting the market growth of refractory glaucoma treatment devices in emerging nations where cataract surgery is the largely performed eye surgery. With new devices and technology being introduced, there could be a strong wave of growth experienced in the North America and European markets.
Iridex Corporation, a global provider of innovative laser systems, device and consumables in ophthalmology announced at the European Glaucoma Society held in June 2016 about the positive results of its MicroPulse P3 (MP3) device. The device powered with MicroPulse laser technology showed long term benefits for patients with refractory glaucoma. Few detailed studies with successful outcomes could lead global acceptance of this technology in future.
Glaucoma drainage devices are frequently used in refractory glaucoma to reduce the intra-ocular pressure (IOC). These devices are available in either with IOP regulating valves or without it. Ahmed glaucoma valve (AGV) is the most commonly used valve-based drainage device while Molteno, Baerveldt, Shocket, and Eagle Vision implants are the non-valved drainage devices available in the Refractory Glaucoma Treatment Market.
About Coherent Market Insights:
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us:
Mr. Shah
Coherent Market Insights
1001 4th Ave,
#3200
Seattle, WA 98154
Tel: +1-206-701-6702
Email: [email protected]
#Refractory Glaucoma Treatment Market#Refractory Glaucoma Treatment Market Size#Refractory Glaucoma Treatment Market Share#Refractory Glaucoma Treatment Market Outlook#Refractory Glaucoma Treatment
0 notes
Text
Macular Edema Treatment Market Showing Compound Annual Growth Rate And Forecast Till 2026
Macular edema is the condition characterized by abnormal leakage and accumulation of fluid that forms blister in the layer of macula. The macula is an oval-shaped pigmented area near the center of retina. This blister causes the macula to swell and thicken that causes distortion of vision. Although macular edema has numerous causes, the most common cause is diabetes. Other causes include, age-related macular degeneration, replacement of lenses in treatment of cataract, chronic uveitis and intermediate uveitis, blockage of vein in the retina, and congenital diseases including retinitis pigmentosa and retinoschisis. Symptoms of macular edema include blurry or wavy vision near or in the center of field of vision. The disease is diagnosed by performing tests such as visual acuity test, dilated eye exam, fluorescein angiogram, optical coherence tomography, and Amsler grid.
Get Exclusive Sample Report at: http://bit.ly/2IgbA7y
Macular Edema Treatment Market: Drivers
Increasing number of pipeline studies is expected to drive the macular edema treatment market growth. For instance, in April 2018, University of California, Davis, in collaboration with IRIDEX Corporation, initiated phase 3 clinical trial for Micropulse Laser Treatment to evaluate micropulse for suppression of diabetic macular edema (pulse). The primary aim of the study is to determine whether the micropulse laser treatment in eyes with good visual acuity will improve or stabilize vision loss due to the complications of diabetic macular edema. The study is expected to be completed by December 2020.
Furthermore, increasing approval for new diabetic macular edema (DME) therapies is expected to propel growth for the macular edema treatment market. For instance, in March 2018, U.S. Food and Drug Administration (FDA) approved Lucentis (ranibizumab injection) 0.3 mg prefilled syringe (PFS) for diabetic macular edema and diabetic retinopathy. Lucentis 0.3 mg PFS is the first syringe prefilled with an anti-vascular endothelial growth factor (VEGF) agent. Moreover, in 2016 U.S.FDA approved Lucentis 0.5 mg PFS, which is intended for use in the treatment of neovascular (wet) age-related macular degeneration (AMD), macular edema following retinal vein occlusion (RVO), and myopic choroidal neovascularisation (mCNV).
However, lack of awareness of diabetic eye diseases among the patients with diabetes is expected to hinder the macular edema treatment market growth.
Macular Edema Treatment Market: Regional Analysis
On the basis of region, the global macular edema treatment market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa.
North America is expected to account for the largest market share in the macular edema treatment market, owing to increasing prevalence of diabetic macular edema in this region. For instance, according to American Journal of Managed Care (AJMC), in 2016, the prevalence rate of diabetic macular edema was 45.3% in North America.
Moreover, in 2013, as per National Center for Biotechnology Information (NCBI), around 98 million individuals reported for diabetic retinopathy (DR) and 21 million individual reported for diabetic macular edema (DME) worldwide.
Furthermore, increasing product approvals for the treatment of macular edema is expected to boost growth of the macular edema treatment market in Europe. For instance, in 2015, the European Commission approved Eylea (aflibercept) for the treatment of patients with macular edema secondary to retinal vein occlusion. Bayer HealthCare and Regeneron collaborated to develop Eylea. Bayer HealthCare has exclusive marketing rights to the product outside the U.S.
Ask For Discount Before Purchasing This Business Report @ http://bit.ly/31wHqo1
Key players operating in the macular edema treatment market include, Novartis International AG, F. Hoffmann-La Roche Ltd, Regeneron Pharmaceuticals, Inc., Pfizer Inc. Bayer AG, Aerpio Therapeutics, Inc., Biomar Microbial Technologies, Antisense Therapeutics Limited, Coherus BioSciences, and Bausch & Lomb Incorporated.
Macular Edema Treatment Market Taxonomy
By Disease Type
Diabetic Macular Edema
Cystoid Macular Edema
By Drug Class
Anti-Vascular endothelial growth factor (VEGF) injections
Avastin
Eylea
Lucentis
Anti-inflammatory Medication
Nonsteroidal anti-inflammatory drugs (NSAIDs)
Corticosteroid
Ozurdex
Retisert
Iluvien
By End User
Hospitals
Ophthalmic Clinics
Research Institutes
By Region
North America
Europe
Asia Pacific
Latin America
Middle East
Africa
About Coherent Market Insights
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us
Mr. Shah
Coherent Market Insights
1001 4th Ave, #3200
Seattle, WA 98154
Tel: +1-206-701-6702
Email: [email protected]
0 notes