#( j.queue )
Photo
New Post has been published on https://fitnesshealthyoga.com/at-2-million-priciest-ever-medicine-treats-fatal-genetic-disease/
At $2 million, priciest ever medicine treats fatal genetic disease
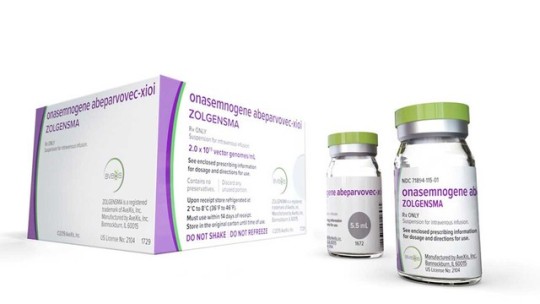
This photo provided by Novartis shows Zolgensma. The one-time gene therapy developed by Novartis, Zolgensma, will cost $2.125 million. It treats a rare condition called spinal muscular atrophy, or SMA, which strikes about 400 babies born in the U.S. each year. The therapy, given in a one-hour infusion, was approved for children under age 2 and will be available within two weeks. (Novartis via AP)
Copyright 2019 Nexstar Broadcasting, Inc. All rights reserved. This material may not be published, broadcast, rewritten, or redistributed.
This photo provided by Novartis shows Zolgensma. The one-time gene therapy developed by Novartis, Zolgensma, will cost $2.125 million. It treats a rare condition called spinal muscular atrophy, or SMA, which strikes about 400 babies born in the U.S. each year. The therapy, given in a one-hour infusion, was approved for children under age 2 and will be available within two weeks. (Novartis via AP)
U.S. regulators have approved the most expensive medicine ever, for a rare disorder that destroys a baby’s muscle control and kills nearly all of those with the most common type of the disease within a couple of years.
The treatment is priced at $2.125 million. Out-of-pocket costs for patients will vary based on insurance coverage.
The medicine, sold by the Swiss drugmaker Novartis, is a gene therapy that treats an inherited condition called spinal muscular atrophy. The treatment targets a defective gene that weakens a child’s muscles so dramatically that they become unable to move, and eventually unable to swallow or breathe. It strikes about 400 babies born in the U.S. each year.
The Food and Drug Administration on Friday approved the treatment, called Zolgensma, for all children under age 2 who are confirmed by a genetic test to have any of the three types of the disease. The therapy is a one-time infusion that takes about an hour.
Novartis said it will let insurers make payments over five years, at $425,000 per year, and will give partial rebates if the treatment doesn’t work.
The one other medicine for the disease approved in the U.S. is a drug called Spinraza. Instead of a one-time treatment, it must be given every four months. Biogen, Spinraza’s maker, charges a list price of $750,000 for the first year and then $350,000 per year after that.
The independent nonprofit group Institute for Clinical and Economic Review, which rates the value of expensive new medicines, calculated that the price of the new gene therapy is justifiable at a cost of $1.2 million to $2.1 million because it “dramatically transforms the lives of families affected by this devastating disease.”
ICER’s president, Dr. Steven D. Pearson, called the treatment’s price “a positive outcome for patients and the entire health system.”
The defective gene that causes spinal muscular atrophy prevents the body from making enough of a protein that allows nerves that control movement to work normally. The nerves die off without the protein.
In the most common type, which is also the most severe, at least 90% of patients die by age 2, and any still alive need a ventilator to breathe. Children with less-severe types become disabled more slowly and can live for up to a couple decades.
Zolgensma works by supplying a healthy copy of the faulty gene, which allows nerve cells to then start producing the needed protein. That halts deterioration of the nerve cells and allows the baby to develop more normally.
In patient testing, babies with the most severe form of the disease who got Zolgensma within 6 months of birth had limited muscle problems. Those who got the treatment earliest did best.
Babies given Zolgensma after six months stopped losing muscle control, but the medicine can’t reverse damage already done.
Evelyn Villarreal was one of the first children treated, at eight weeks. Her family, from Centreville, Virginia, had lost their first child to spinal muscular atrophy at 15 months. Two years later when Evelyn was born a test showed she also had the disease, so the family enrolled her in the gene therapy study at Nationwide Children’s Hospital in Columbus, Ohio.
Evelyn is now 4½ years old and showing no muscle problems other than minor trouble standing up, said her mother, Elena Villarreal. She has been feeding herself for a long time, she draws and speaks well, and will be starting kindergarten in the fall.
“She’s very active and goes to the playground a lot,” said Elena Villarreal. “She’s walking and even jumping.”
It is too early to know how long the benefit of the treatment lasts, but doctors’ hopes are rising that they could last a lifetime, according to Dr. Jerry Mendell, a neurologist at Nationwide Children’s. Mendell led one of the early patient studies and is Evelyn’s doctor.
“It’s beginning to look that way,” he said, because a few children treated who are now 4 or 5 still have no symptoms.
Early diagnosis is crucial, so Novartis has been working with states to get genetic testing for newborns required at birth. It expects most states will have that requirement by next year.
The FDA said side effects included vomiting and potential liver damage, so patients must be monitored for the first few months after treatment.
___
Follow Linda A. Johnson at https://twitter.com/LindaJ_onPharma .
___
The Associated Press Health and Science Department receives supportfrom the Howard Hughes Medical Institute’s Department of Science Education. The AP is solely responsible for all content.
(function(e,a,f)var c,b=e.getElementsByTagName(a)[0];if(e.getElementById(f))returnc=e.createElement(a);c.id=f;c.src="http://connect.facebook.net/en_US/sdk.js#xfbml=1&version=v2.5";b.parentNode.insertBefore(c,b)(document,"script","facebook-jssdk"));!function(h,a,i,c,j,d,g)if(h.fbq)returnj=h.fbq=function()j.callMethod?j.callMethod.apply(j,arguments):j.queue.push(arguments);if(!h._fbq)h._fbq=jj.push=j;j.loaded=!0;j.version="2.0";j.queue=[];d=a.createElement(i);d.async=!0;d.src=c;g=a.getElementsByTagName(i)[0];g.parentNode.insertBefore(d,g)(window,document,"script","https://connect.facebook.net/en_US/fbevents.js");fbq("init","167243700318082");fbq("track","PageView");
Source link
#current health news usa#Facebook-Instant#Home#latest health news usa#News#U.S. & World#us public health news#Health News
0 notes
Photo

New Post has been published on https://fitnesshealthyoga.com/virus-halts-dog-adoptions-in-clark-county-for-2-weeks/
Virus halts dog adoptions in Clark County for 2 weeks
Copyright 2018 Nexstar Broadcasting, Inc. All rights reserved. This material may not be published, broadcast, rewritten, or redistributed.
SPRINGFIELD, Ohio (WDTN) – The Clark County Dog Shelter said Friday it will not be conducting adoptions for the next two weeks due to an outbreak of a contagious canine virus.
“The top priority at the Clark County Dog Shelter is always to ensure the health and well-being of the dogs we care for,” said Dog Warden Sandi Click. “The decision to close is in keeping with the recommendations of four different veterinary practices here in Clark County. We have a protocol in place for incoming dogs during this time and wish to protect the dogs currently in our care from undue stress.”
The dog shelter will continue the redemption process by appointment throughout the 14-day period. It will continue to update its Facebook page with strays that were impounded in order to help reunite them with their owner, per the Ohio Revised Code. It will resume performing adoptions on Friday, Jan. 11.
The shelter says several dogs in Clark County have been diagnosed with canine parvovirus, a contagious disease that can affect all dogs, but particularly unvaccinated dogs and puppies. Officials say the shelter recently saw one case of parvovirus. Symptoms include lethargy, loss of appetite, bloating, fever, low body temperature, vomiting and severe or bloody diarrhea, according to the American Veterinary Medical Association.
Proper disinfection of contaminated kennels is essential to control the spread of parvovirus. The virus is not easily killed and the AMVA recommends consulting your veterinarian for specific guidance on cleaning and disinfecting agents. Vaccination and proper hygiene procedures are recommended to prevent the virus. Canine parvovirus cannot be contracted by humans.
For more information about parvovirus, click here.
Grab the FREE WDTN News App for iPhone or Android. Stay up to date with all the local news, weather and sports as well as live newscasts and events as they happen.
Like us on Facebook and follow us on Twitter for all the latest news, weather and sports.
!function(h,a,i,c,j,d,g)if(h.fbq)returnj=h.fbq=function()j.callMethod?j.callMethod.apply(j,arguments):j.queue.push(arguments);if(!h._fbq)h._fbq=jj.push=j;j.loaded=!0;j.version="2.0";j.queue=[];d=a.createElement(i);d.async=!0;d.src=c;g=a.getElementsByTagName(i)[0];g.parentNode.insertBefore(d,g)(window,document,"script","https://connect.facebook.net/en_US/fbevents.js");fbq("init","382472938939759");fbq("track","PageView");
Source link
#As Seen on 2 NEWS#Don&039;t Miss#Facebook-Instant#health insurance news usa#health news united states#health news usa#Local News#News#Top Stories#Health News
0 notes